Detergent-extracted Volvox model exhibits an anterior-posterior gradient in flagellar Ca2+ sensitivity
- PMID: 29311312
- PMCID: PMC5798354
- DOI: 10.1073/pnas.1715489115
Detergent-extracted Volvox model exhibits an anterior-posterior gradient in flagellar Ca2+ sensitivity
Abstract
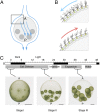
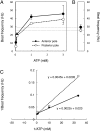
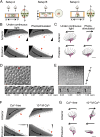
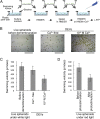
Volvox rousseletii is a multicellular spheroidal green alga containing ∼5,000 cells, each equipped with two flagella (cilia). This organism shows striking photobehavior without any known intercellular communication. To help understand how the behavior of flagella is regulated, we developed a method to extract the whole organism with detergent and reactivate its flagellar motility. Upon addition of ATP, demembranated flagella (axonemes) in the spheroids actively beat and the spheroids swam as if they were alive. Under Ca2+-free conditions, the axonemes assumed planar and asymmetrical waveforms and beat toward the posterior pole, as do live spheroids in the absence of light stimulation. In the presence of 10-6 M Ca2+, however, most axonemes beat three-dimensionally toward the anterior pole, similar to flagella in photostimulated live spheroids. This Ca2+-dependent change in flagellar beating direction was more conspicuous near the anterior pole of the spheroid, but was not observed near the posterior pole. This anterior-posterior gradient of flagellar Ca2+ sensitivity may explain the mechanism of V. rousseletii photobehavior.
Keywords: Volvox; calcium; flagella; phototaxis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
How 5000 independent rowers coordinate their strokes in order to row into the sunlight: phototaxis in the multicellular green alga Volvox.BMC Biol. 2010 Jul 27;8:103. doi: 10.1186/1741-7007-8-103. BMC Biol. 2010. PMID: 20663212 Free PMC article.
-
Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas.J Cell Biol. 1984 Jan;98(1):97-107. doi: 10.1083/jcb.98.1.97. J Cell Biol. 1984. PMID: 6707098 Free PMC article.
-
Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas.J Cell Biol. 1980 Aug;86(2):446-55. doi: 10.1083/jcb.86.2.446. J Cell Biol. 1980. PMID: 6447155 Free PMC article.
-
How are different ciliary beat patterns produced?Symp Soc Exp Biol. 1982;35:139-57. Symp Soc Exp Biol. 1982. PMID: 6223395 Review.
-
New insights into the roles of molecular chaperones in Chlamydomonas and Volvox.Int Rev Cell Mol Biol. 2010;285:75-113. doi: 10.1016/B978-0-12-381047-2.00002-5. Int Rev Cell Mol Biol. 2010. PMID: 21035098 Review.
Cited by
-
Volvox and volvocine green algae.Evodevo. 2020 Jul 1;11:13. doi: 10.1186/s13227-020-00158-7. eCollection 2020. Evodevo. 2020. PMID: 32626570 Free PMC article. Review.
-
The 57th Annual Meeting of the Biophysical Society of Japan : Session 3SCA - Diversity and universality of motile mechanism of living things: from intracellular dynamics to collective motion.Biophys Rev. 2020 Apr;12(2):293-294. doi: 10.1007/s12551-020-00629-0. Epub 2020 Jan 29. Biophys Rev. 2020. PMID: 31993936 Free PMC article. No abstract available.
-
Intermediate light adaptation induces oscillatory phototaxis switching and pattern formation in Chlamydomonas.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2425369122. doi: 10.1073/pnas.2425369122. Epub 2025 Jun 12. Proc Natl Acad Sci U S A. 2025. PMID: 40504160
-
Swimming ability and flagellar motility of sperm packets of the volvocine green alga Pleodorina starrii.PLoS One. 2024 Jul 18;19(7):e0287561. doi: 10.1371/journal.pone.0287561. eCollection 2024. PLoS One. 2024. PMID: 39024288 Free PMC article.
-
Multicellularity and increasing Reynolds number impact on the evolutionary shift in flash-induced ciliary response in Volvocales.BMC Ecol Evol. 2024 Sep 14;24(1):119. doi: 10.1186/s12862-024-02307-1. BMC Ecol Evol. 2024. PMID: 39277710 Free PMC article.
References
-
- Solari CA, Michod RE, Goldstein RE. Volvox barberi, the fastest swimmer of the Volvocales (Chlorophyceae) J Phycol. 2008;44:1395–1398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

