Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes
- PMID: 29311338
- PMCID: PMC5789931
- DOI: 10.1073/pnas.1715865115
Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes
Erratum in
-
Correction for Conforti et al., Faulty neuronal determination and cell polarization are reverted by modulating HD early phenotypes.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):E2148. doi: 10.1073/pnas.1801898115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463700 Free PMC article. No abstract available.
Abstract
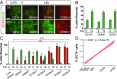
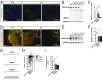
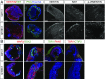
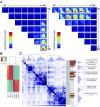
Increasing evidence suggests that early neurodevelopmental defects in Huntington's disease (HD) patients could contribute to the later adult neurodegenerative phenotype. Here, by using HD-derived induced pluripotent stem cell lines, we report that early telencephalic induction and late neural identity are affected in cortical and striatal populations. We show that a large CAG expansion causes complete failure of the neuro-ectodermal acquisition, while cells carrying shorter CAGs repeats show gross abnormalities in neural rosette formation as well as disrupted cytoarchitecture in cortical organoids. Gene-expression analysis showed that control organoid overlapped with mature human fetal cortical areas, while HD organoids correlated with the immature ventricular zone/subventricular zone. We also report that defects in neuroectoderm and rosette formation could be rescued by molecular and pharmacological approaches leading to a recovery of striatal identity. These results show that mutant huntingtin precludes normal neuronal fate acquisition and highlights a possible connection between mutant huntingtin and abnormal neural development in HD.
Keywords: Huntington’s disease; human iPS lines; neurodevelopment; organoids; striatal differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cudkowicz M, Kowall NW. Degeneration of pyramidal projection neurons in Huntington’s disease cortex. Ann Neurol. 1990;27:200–204. - PubMed
-
- Duyao MP, et al. Inactivation of the mouse Huntington’s disease gene homolog Hdh. Science. 1995;269:407–410. - PubMed
-
- Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat Genet. 1995;11:155–163. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

