Yolk sac macrophage progenitors traffic to the embryo during defined stages of development
- PMID: 29311541
- PMCID: PMC5758709
- DOI: 10.1038/s41467-017-02492-2
Yolk sac macrophage progenitors traffic to the embryo during defined stages of development
Erratum in
-
Author Correction: Yolk sac macrophage progenitors traffic to the embryo during defined stages of development.Nat Commun. 2018 Sep 7;9(1):3699. doi: 10.1038/s41467-018-06065-9. Nat Commun. 2018. PMID: 30194428 Free PMC article.
Abstract
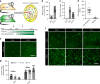
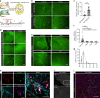
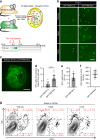
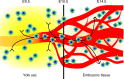
Tissue macrophages in many adult organs originate from yolk sac (YS) progenitors, which invade the developing embryo and persist by means of local self-renewal. However, the route and characteristics of YS macrophage trafficking during embryogenesis are incompletely understood. Here we show the early migration dynamics of YS-derived macrophage progenitors in vivo using fate mapping and intravital microscopy. From embryonic day 8.5 (E8.5) CX3CR1+ pre-macrophages are present in the mouse YS where they rapidly proliferate and gain access to the bloodstream to migrate towards the embryo. Trafficking of pre-macrophages and their progenitors from the YS to tissues peaks around E10.5, dramatically decreases towards E12.5 and is no longer evident from E14.5 onwards. Thus, YS progenitors use the vascular system during a restricted time window of embryogenesis to invade the growing fetus. These findings close an important gap in our understanding of the development of the innate immune system.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
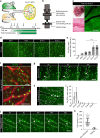
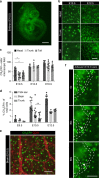
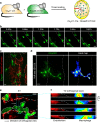
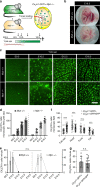
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

