Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle
- PMID: 29311556
- PMCID: PMC5758577
- DOI: 10.1038/s41467-017-02591-0
Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle
Abstract
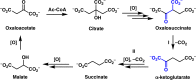
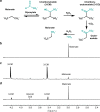
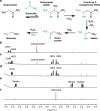
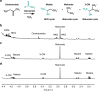
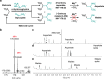
The development of metabolic approaches towards understanding the origins of life, which have focused mainly on the citric acid (TCA) cycle, have languished-primarily due to a lack of experimentally demonstrable and sustainable cycle(s) of reactions. We show here the existence of a protometabolic analog of the TCA involving two linked cycles, which convert glyoxylate into CO2 and produce aspartic acid in the presence of ammonia. The reactions proceed from either pyruvate, oxaloacetate or malonate in the presence of glyoxylate as the carbon source and hydrogen peroxide as the oxidant under neutral aqueous conditions and at mild temperatures. The reaction pathway demonstrates turnover under controlled conditions. These results indicate that simpler versions of metabolic cycles could have emerged under potential prebiotic conditions, laying the foundation for the appearance of more sophisticated metabolic pathways once control by (polymeric) catalysts became available.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Dyson, F. Origins of Life (Cambridge University Press, Cambridge, 1999).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

