Multi-day rhythms modulate seizure risk in epilepsy
- PMID: 29311566
- PMCID: PMC5758806
- DOI: 10.1038/s41467-017-02577-y
Multi-day rhythms modulate seizure risk in epilepsy
Abstract
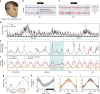
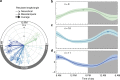
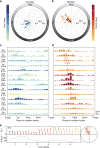
Epilepsy is defined by the seemingly random occurrence of spontaneous seizures. The ability to anticipate seizures would enable preventative treatment strategies. A central but unresolved question concerns the relationship of seizure timing to fluctuating rates of interictal epileptiform discharges (here termed interictal epileptiform activity, IEA), a marker of brain irritability observed between seizures by electroencephalography (EEG). Here, in 37 subjects with an implanted brain stimulation device that detects IEA and seizures over years, we find that IEA oscillates with circadian and subject-specific multidien (multi-day) periods. Multidien periodicities, most commonly 20-30 days in duration, are robust and relatively stable for up to 10 years in men and women. We show that seizures occur preferentially during the rising phase of multidien IEA rhythms. Combining phase information from circadian and multidien IEA rhythms provides a novel biomarker for determining relative seizure risk with a large effect size in most subjects.
Conflict of interest statement
V.R.R. and D.K.-S. have received honoraria from NeuroPace, Inc. for consulting and speaking engagements. E.A.M. is an employee of NeuroPace, Inc. M.O.B. is a part-time employee of the Wyss Center for Bio and Neuroengineering. The authors declare no targeted funding or compensation from NeuroPace, Inc. for this study. The remaining authors declare no competing financial interests.
Figures



Comment in
-
A Lunatic Dance: Circadian and Multidien Structures of Seizure Timing.Epilepsy Curr. 2018 May-Jun;18(3):194-196. doi: 10.5698/1535-7597.18.3.194. Epilepsy Curr. 2018. PMID: 29950949 Free PMC article. No abstract available.
-
Long-Term Patterns of Seizure Recurrence: Estimating Risk From Ambulatory Intracranial EEG Recordings.Epilepsy Curr. 2018 Jul-Aug;18(4):236-237. doi: 10.5698/1535-7597.18.4.236. Epilepsy Curr. 2018. PMID: 30254518 Free PMC article. No abstract available.
References
-
- Temkin, O. The falling sickness: A history of epilepsy from the Greeks to the beginnings of modern neurology. (Johns Hopkins University Press, 1994).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

