From stable Sb- and Bi-centered radicals to a compound with a Ga=Sb double bond
- PMID: 29311607
- PMCID: PMC5758792
- DOI: 10.1038/s41467-017-02581-2
From stable Sb- and Bi-centered radicals to a compound with a Ga=Sb double bond
Abstract
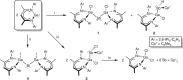
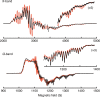
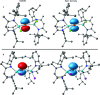
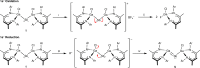
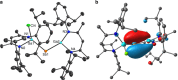
Neutral stibinyl and bismuthinyl radicals are typically short-lived, reactive species. Here we show the synthesis and solid-state structures of two stable stibinyl [L(Cl)Ga]2Sb· 1 and bismuthinyl radicals [L(I)Ga]2Bi· 4, which are stabilized by electropositive metal centers. Their description as predominantly metal-centered radicals is consistent with the results of NMR, EPR, SQUID, and DFT studies. The Lewis-acidic character of the Ga ligands allow for significant electron delocalization of the Sb- and Bi- unpaired radical onto the ligand. Single-electron reduction of [L(Cl)Ga]2Sb· gave LGaSbGa(Cl)L 5, the first compound containing a Ga=Sb double bond. The π-bonding contribution is estimated to 9.56 kcal mol-1 by NMR spectroscopy. The bonding situation and electronic structure is analyzed by quantum mechanical computations, revealing significant π backdonation from the Sb to the Ga atom. The formation of 5 illustrates the high-synthetic potential of 1 for the formation of new compounds with unusual electronic structures.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Gomberg M. An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 1900;22:757–771. doi: 10.1021/ja02049a006. - DOI
-
- Breher F. Stretching bonds in main group element compounds - Borderlines between biradicals and closed-shell species. Coord. Chem. Rev. 2007;251:1007–1043. doi: 10.1016/j.ccr.2006.09.007. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

