TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD
- PMID: 29311743
- PMCID: PMC5800968
- DOI: 10.1038/s41593-017-0047-3
TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD
Abstract
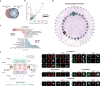
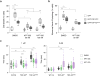
The cytoplasmic mislocalization and aggregation of TAR DNA-binding protein-43 (TDP-43) is a common histopathological hallmark of the amyotrophic lateral sclerosis and frontotemporal dementia disease spectrum (ALS/FTD). However, the composition of aggregates and their contribution to the disease process remain unknown. Here we used proximity-dependent biotin identification (BioID) to interrogate the interactome of detergent-insoluble TDP-43 aggregates and found them enriched for components of the nuclear pore complex and nucleocytoplasmic transport machinery. Aggregated and disease-linked mutant TDP-43 triggered the sequestration and/or mislocalization of nucleoporins and transport factors, and interfered with nuclear protein import and RNA export in mouse primary cortical neurons, human fibroblasts and induced pluripotent stem cell-derived neurons. Nuclear pore pathology is present in brain tissue in cases of sporadic ALS and those involving genetic mutations in TARDBP and C9orf72. Our data strongly implicate TDP-43-mediated nucleocytoplasmic transport defects as a common disease mechanism in ALS/FTD.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






Comment in
-
Neurodegenerative disease: TDP pathology leads to nuclear disruption.Nat Rev Neurol. 2018 Mar;14(3):126-127. doi: 10.1038/nrneurol.2018.2. Epub 2018 Jan 19. Nat Rev Neurol. 2018. PMID: 29348541 No abstract available.
-
Nuclear pores: the gate to neurodegeneration.Nat Neurosci. 2018 Feb;21(2):156-158. doi: 10.1038/s41593-017-0066-0. Nat Neurosci. 2018. PMID: 29371653 No abstract available.
References
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. - PubMed
-
- Buratti E, et al. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

