Hippocampal Neurodegenerative Pathology in Post-stroke Dementia Compared to Other Dementias and Aging Controls
- PMID: 29311794
- PMCID: PMC5742173
- DOI: 10.3389/fnins.2017.00717
Hippocampal Neurodegenerative Pathology in Post-stroke Dementia Compared to Other Dementias and Aging Controls
Abstract
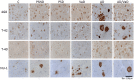
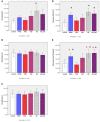
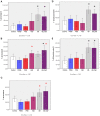
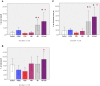
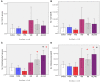
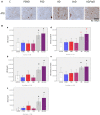
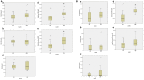
Neuroimaging evidence from older stroke survivors in Nigeria and Northeast England showed medial temporal lobe atrophy (MTLA) to be independently associated with post-stroke cognitive impairment and dementia. Given the hypothesis ascribing MTLA to neurodegenerative processes, we assessed Alzheimer pathology in the hippocampal formation and entorhinal cortex of autopsied brains from of post-stroke demented and non-demented subjects in comparison with controls and other dementias. We quantified markers of amyloid β (total Aβ, Aβ-40, Aβ-42, and soluble Aβ) and hyperphosphorylated tau in the hippocampal formation and entorhinal cortex of 94 subjects consisting of normal controls (n = 12), vascular dementia, VaD (17), post-stroke demented, PSD (n = 15), and post-stroke non-demented, PSND (n = 23), Alzheimer's disease, AD (n = 14), and mixed AD and vascular dementia, AD_VAD (n = 13) using immunohistochemical techniques. We found differential expression of amyloid and tau across the disease groups, and across hippocampal sub-regions. Among amyloid markers, the pattern of Aβ-42 immunoreactivity was similar to that of total Aβ. Tau immunoreactivity showed highest expression in the AD and mixed AD and vascular dementia, AD_VaD, which was higher than in control, post - stroke and VaD groups (p < 0.05). APOE ε4 allele positivity was associated with higher expression of amyloid and tau pathology in the subiculum and entorhinal cortex of post-stroke cases (p < 0.05). Comparison between PSND and PSD revealed higher total Aβ immunoreactivity in PSND compared to PSD in the CA1, subiculum and entorhinal cortex (p < 0.05) but no differences between PSND and PSD in Aβ-42, Aβ-40, soluble Aβ or tau immunoreactivities (p > 0.05). Correlation of MMSE and CAMCOG scores with AD pathological measures showed lack of correlation with amyloid species although tau immunoreactivity demonstrated correlation with memory scores (p < 0.05). Our findings suggest hippocampal AD pathology does not necessarily differ between demented and non-demented post-stroke subjects. The dissociation of cognitive performance with hippocampal AD pathological burden suggests more dominant roles for non-Alzheimer neurodegenerative and / or other non-neurodegenerative substrates for dementia following stroke.
Keywords: Alzheimer's disease; cerebrovascular disease; mixed dementia; post-stroke dementia; stroke; vascular dementia.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

