Palmitoylethanolamide Supplementation during Sensitization Prevents Airway Allergic Symptoms in the Mouse
- PMID: 29311913
- PMCID: PMC5732963
- DOI: 10.3389/fphar.2017.00857
Palmitoylethanolamide Supplementation during Sensitization Prevents Airway Allergic Symptoms in the Mouse
Abstract
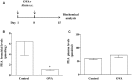
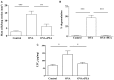
One important risk factor for the development of asthma is allergen sensitization. Recent increasing evidence suggests a prominent role of mast cells in asthma pathophysiology. Since Palmitoylethanolamide (PEA), an endogenous lipid mediator chemically related to - and co-released with- the endocannabinoid anandamide, behaves as a local autacoid down-regulator of mast cell activation and inflammation, we explored the possible contribution of PEA in allergic sensitization, by using ovalbumin (OVA) as sensitizing agent in the mouse. PEA levels were dramatically reduced in the bronchi of OVA-treated animals. This effect was coupled to a significant up-regulation of CB2 and GPR55 receptors, two of the proposed molecular PEA targets, in bronchi harvested from allergen-sensitized mice. PEA supplementation (10 mg/kg, 15 min before each allergen exposure) prevented OVA-induced bronchial hyperreactivity, but it did not affect IgE plasma increase. On the other hand, PEA abrogated allergen-induced cell recruitment as well as pulmonary inflammation. Evaluation of pulmonary sections evidenced a significant inhibitory action of PEA on pulmonary mast cell recruitment and degranulation, an effect coupled to a reduction of leukotriene C4 production. These findings demonstrate that allergen sensitization negatively affects PEA bronchial levels and suggest that its supplementation has the potential to prevent the development of asthma-like features.
Keywords: airway inflammation; allergen sensitization; bronchial hyperreactivity; mast cells; palmitoylethanolamide.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

