Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in Vitro and in Vivo
- PMID: 29311932
- PMCID: PMC5742161
- DOI: 10.3389/fphar.2017.00929
Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in Vitro and in Vivo
Abstract
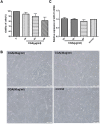
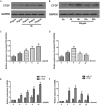
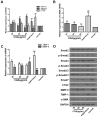
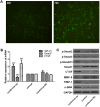
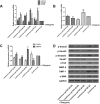
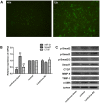
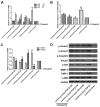
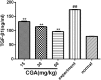
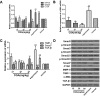
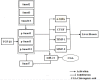
Aims: Chlorogenic acid (CGA) is a phenolic acid that has a wide range of pharmacological effects. However, the protective effects and mechanisms of CGA on liver fibrosis are not clear. This study explored the effects of CGA on miR-21-regulated TGF-β1/Smad7 liver fibrosis in the hepatic stellate LX2 cell line and in CCl4-induced liver fibrosis in Sprague-Dawley rats. Methods: The mRNA expression of miR-21, Smad7, connective tissue growth factor (CTGF), α-smooth muscle actin (α-SMA), tissue inhibitor of metalloproteinase 1 (TIMP-1), matrix metalloproteinase-9 (MMP-9), and transforming growth factor-β1 (TGF-β1) and the protein levels of Smad2, p-Smad2, Smad3, p-Smad3, Smad2/3, p-Smad2/3, Smad7, CTGF, α-SMA, TIMP-1, MMP-9 and TGF-β1 were assayed in LX2 cells and liver tissue. The effects of CGA after miR-21 knockdown or overexpression were analyzed in LX2 cells. The liver tissue and serum were collected for histopathological examination, immunohistochemistry (IHC) and ELISA. Results: The mRNA expression of miR-21, CTGF, α-SMA, TIMP-1, and TGF-β1 and the protein expression of p-Smad2, p-Smad3, p-Smad2/3, CTGF, α-SMA, TIMP-1, and TGF-β1 were inhibited by CGA both in vitro and in vivo. Meanwhile, CGA elevated the mRNA and protein expression of Smad7 and MMP-9. After miR-21 knockdown and overexpression, the downstream molecules also changed accordingly. CGA also lessened the degree of liver fibrosis in the pathological manifestation and reduced α-SMA and collagen I expression in liver tissue and TGF-β1 in serum. Conclusion: CGA might relieve liver fibrosis through the miR-21-regulated TGF-β1/Smad7 signaling pathway, which suggests that CGA might be a new anti-fibrosis agent that improves liver fibrosis.
Keywords: Smad7; TGF-β1; chlorogenic acid; liver fibrosis; miR-21.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

