Characterizing Isozymes of Chlorite Dismutase for Water Treatment
- PMID: 29312158
- PMCID: PMC5733030
- DOI: 10.3389/fmicb.2017.02423
Characterizing Isozymes of Chlorite Dismutase for Water Treatment
Abstract
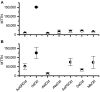
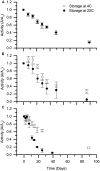
This work investigated the potential for biocatalytic degradation of micropollutants, focusing on chlorine oxyanions as model contaminants, by mining biology to identify promising biocatalysts. Existing isozymes of chlorite dismutase (Cld) were characterized with respect to parameters relevant to this high volume, low-value product application: kinetic parameters, resistance to catalytic inactivation, and stability. Maximum reaction velocities (Vmax) were typically on the order of 104 μmol min-1 (μmol heme)-1. Substrate affinity (Km) values were on the order of 100 μM, except for the Cld from Candidatus Nitrospira defluvii (NdCld), which showed a significantly lower affinity for chlorite. NdCld also had the highest susceptibility to catalytic inactivation. In contrast, the Cld from Ideonella dechloratans was least susceptible to catalytic inactivation, with a maximum turnover number of approximately 150,000, more than sevenfold higher than other tested isozymes. Under non-reactive conditions, Cld was quite stable, retaining over 50% of activity after 30 days, and most samples retained activity even after 90-100 days. Overall, Cld from I. dechloratans was the most promising candidate for environmental applications, having high affinity and activity, a relatively low propensity for catalytic inactivation, and excellent stability.
Keywords: biocatalyst; catalytic inactivation; chlorite; drinking water; perchlorate.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

