A Single-Nucleotide Deletion in the Transcription Factor Gene bcsmr1 Causes Sclerotial-Melanogenesis Deficiency in Botrytis cinerea
- PMID: 29312200
- PMCID: PMC5733056
- DOI: 10.3389/fmicb.2017.02492
A Single-Nucleotide Deletion in the Transcription Factor Gene bcsmr1 Causes Sclerotial-Melanogenesis Deficiency in Botrytis cinerea
Abstract
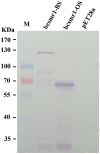
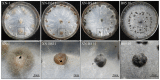
Botrytis cinerea is an important plant pathogenic fungus with a wide range of host. It usually produces black-colored sclerotia (BS) due to deposition of 1,8-dihydroxynaphthalene melanin in sclerotial melanogenesis. Our previous study (Zhou et al., 2018) reported six B. cinerea isolates producing orange-colored sclerotia (OS) with deficiency in sclerotial melanogenesis. Comparison of ecological fitness (conidia, mycelia, sclerotia), natural distribution, and melanogenesis of selected BS and OS isolates suggests that sclerotia play an important role in the disease cycle caused by B. cinerea. However, the molecular mechanism for formation of the OS B. cinerea remains unknown. This study was done to unravel the molecular mechanism for the sclerotial melanogenesis deficiency in the OS isolates. We found that all the five sclerotial melanogenesis genes (bcpks12, bcygh1, bcbrn1/2, bcscd1) were down-regulated in OS isolates, compared to the genes in the BS isolates. However, the sclerotial melanogenesis-regulatory gene bcsmr1 had similar expression in both types of sclerotia, suggesting the sclerotial melanogenesis deficiency is due to loss-of-function of bcsmr1, rather than lack of expression of bcsmr1. Therefore, we cloned bcsmr1 from OS (bcsmr1OS ) and BS (bcsmr1BS ) isolates, and found a single-nucleotide deletion in bcsmr1OS . The single-nucleotide deletion caused formation of a premature stop codon in the open reading frame of bcsmr1OS , resulting in production of a 465-aa truncated protein. The transcription activity of the truncated protein was greatly reduced, compared to that of the 935-aa full-length protein encoded by bcsmr1BS in the BS isolates. The function of bcsmr1OS was partially complemented by bcsmr1BS . This study not only elucidated the molecular mechanism for formation of orange-colored sclerotia by the spontaneous mutant XN-1 of B. cinerea, but also confirmed the regulatory function of bcsmr1 in sclerotial melanogenesis of B. cinerea.
Keywords: Botrytis cinerea; bcsmr1; orange-colored sclerotia; sclerotial melanogenesis; single-nucleotide deletion.
Figures
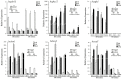



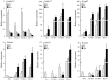
 P < 0.05 and
P < 0.05 and  P < 0.01 (Student's t-test) between each mutant and B05.10.
P < 0.01 (Student's t-test) between each mutant and B05.10.References
-
- Ambrico M. (2016). Special issue: melanin, along lasting history bridging natural pigments and organic bioelectronics. Polym. Int. 65, 1249–1250. 10.1002/pi.5239 - DOI
-
- Bell A. A., Wheeler M. H. (1986). Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24, 411–451. 10.1146/annurev.py.24.090186.002211 - DOI
-
- Butler M. J., Day A. W. (1998). Fungal melanins: a review. Can. J. Microbiol. 44, 1115–1136. 10.1139/w98-119 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

