Autoinducer-2 Quorum Sensing Contributes to Regulation of Microcin PDI in Escherichia coli
- PMID: 29312248
- PMCID: PMC5743794
- DOI: 10.3389/fmicb.2017.02570
Autoinducer-2 Quorum Sensing Contributes to Regulation of Microcin PDI in Escherichia coli
Abstract
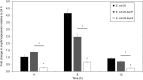
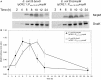
The Escherichia coli quorum sensing (QS) signal molecule, autoinducer-2 (AI-2), reaches its maximum concentration during mid-to-late growth phase after which it quickly degrades during stationary phase. This pattern of AI-2 concentration coincides with the up- then down-regulation of a recently described microcin PDI (mccPDI) effector protein (McpM). To determine if there is a functional relationship between these systems, a prototypical mccPDI-expressing strain of E. coli 25 was used to generate ΔluxS, ΔlsrACDBFG (Δlsr), and ΔlsrR mutant strains that are deficient in AI-2 production, transportation, and AI-2 transport regulation, respectively. Trans-complementation, RT-qPCR, and western blot assays were used to detect changes of microcin expression and synthesis under co-culture and monoculture conditions. Compared to the wild-type strain, the AI-2-deficient strain (ΔluxS) and -uptake negative strain (Δlsr) were >1,000-fold less inhibitory to susceptible bacteria (P < 0.05). With in trans complementation of luxS, the AI-2 deficient mutant reduced the susceptible E. coli population by 4-log, which was within 1-log of the wild-type phenotype. RT-qPCR and western blot results for the AI-2 deficient E. coli 25 showed a 5-fold reduction in mcpM transcription with an average 2-h delay in McpM synthesis. Furthermore, overexpression of sRNA micC and micF (both involved in porin protein regulation) was correlated with mcpM regulation, consistent with a possible link between QS and mcpM regulation. This is the direct first evidence that microcin regulation can be linked to quorum sensing in a Gram-negative bacterium.
Keywords: autoinducer-2; bacteriocin; lsr; luxS; mccPDI; microcin; quorum sensing.
Figures




References
-
- Blanchard A. E., Liao C., Lu T. (2016). An ecological understanding of quorum sensing-controlled bacteriocin synthesis. Cell. Mol. Bioeng. 9, 443–454. 10.1007/s12195-016-0447-6 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

