Synthetic Nanoparticles That Promote Tumor Necrosis Factor Receptor 2 Expressing Regulatory T Cells in the Lung and Resistance to Allergic Airways Inflammation
- PMID: 29312323
- PMCID: PMC5744007
- DOI: 10.3389/fimmu.2017.01812
Synthetic Nanoparticles That Promote Tumor Necrosis Factor Receptor 2 Expressing Regulatory T Cells in the Lung and Resistance to Allergic Airways Inflammation
Abstract
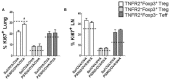
Synthetic glycine coated 50 nm polystyrene nanoparticles (NP) (PS50G), unlike ambient NP, do not promote pulmonary inflammation, but instead, render lungs resistant to the development of allergic airway inflammation. In this study, we show that PS50G modulate the frequency and phenotype of regulatory T cells (Treg) in the lung, specifically increasing the proportion of tumor necrosis factor 2 (TNFR2) expressing Treg. Mice pre-exposed to PS50G, which were sensitized and then challenged with an allergen a month later, preferentially expanded TNFR2+Foxp3+ Treg, which further expressed enhanced levels of latency associated peptide and cytotoxic T-lymphocyte associated molecule-4. Moreover, PS50G-induced CD103+ dendritic cell activation in the lung was associated with the proliferative expansion of TNFR2+Foxp3+ Treg. These findings provide the first evidence that engineered NP can promote the selective expansion of maximally suppressing TNFR2+Foxp3+ Treg and further suggest a novel mechanism by which NP may promote healthy lung homeostasis.
Keywords: PS50G; animal model; asthma; lung; lymph nodes; nanoparticles; tumor necrosis factor 2.
Figures






Similar articles
-
Inert 50-nm polystyrene nanoparticles that modify pulmonary dendritic cell function and inhibit allergic airway inflammation.J Immunol. 2012 Feb 1;188(3):1431-41. doi: 10.4049/jimmunol.1100156. Epub 2011 Dec 21. J Immunol. 2012. PMID: 22190179
-
Differential uptake of nanoparticles and microparticles by pulmonary APC subsets induces discrete immunological imprints.J Immunol. 2013 Nov 15;191(10):5278-90. doi: 10.4049/jimmunol.1203131. Epub 2013 Oct 11. J Immunol. 2013. PMID: 24123688
-
Exacerbation of Ventilation-Induced Lung Injury and Inflammation in Preterm Lambs by High-Dose Nanoparticles.Sci Rep. 2017 Oct 31;7(1):14704. doi: 10.1038/s41598-017-13113-9. Sci Rep. 2017. PMID: 29089616 Free PMC article.
-
IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma.J Immunol. 2015 Feb 1;194(3):887-97. doi: 10.4049/jimmunol.1401612. Epub 2014 Dec 19. J Immunol. 2015. PMID: 25527785
-
Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis.Carcinogenesis. 2013 Jun;34(6):1296-303. doi: 10.1093/carcin/bgt038. Epub 2013 Feb 5. Carcinogenesis. 2013. PMID: 23385062
Cited by
-
The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review.Front Immunol. 2018 Nov 9;9:2572. doi: 10.3389/fimmu.2018.02572. eCollection 2018. Front Immunol. 2018. PMID: 30473698 Free PMC article. Review.
-
A Perspective Review on the Role of Nanomedicine in the Modulation of TNF-TNFR2 Axis in Breast Cancer Immunotherapy.J Oncol. 2019 May 23;2019:6313242. doi: 10.1155/2019/6313242. eCollection 2019. J Oncol. 2019. PMID: 31239840 Free PMC article. Review.
-
Emerging Role of Immunosuppression in Diseases Induced by Micro- and Nano-Particles: Time to Revisit the Exclusive Inflammatory Scenario.Front Immunol. 2018 Nov 19;9:2364. doi: 10.3389/fimmu.2018.02364. eCollection 2018. Front Immunol. 2018. PMID: 30510551 Free PMC article. Review.
-
COVID-19 infection and nanomedicine applications for development of vaccines and therapeutics: An overview and future perspectives based on polymersomes.Eur J Pharmacol. 2021 Apr 5;896:173930. doi: 10.1016/j.ejphar.2021.173930. Epub 2021 Feb 3. Eur J Pharmacol. 2021. PMID: 33545157 Free PMC article. Review.
-
Biomaterial-enhanced treg cell immunotherapy: A promising approach for transplant medicine and autoimmune disease treatment.Bioact Mater. 2024 Apr 22;37:269-298. doi: 10.1016/j.bioactmat.2024.03.030. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 38694761 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

