Accomplices of the Hypoxic Tumor Microenvironment Compromising Antitumor Immunity: Adenosine, Lactate, Acidosis, Vascular Endothelial Growth Factor, Potassium Ions, and Phosphatidylserine
- PMID: 29312351
- PMCID: PMC5742577
- DOI: 10.3389/fimmu.2017.01887
Accomplices of the Hypoxic Tumor Microenvironment Compromising Antitumor Immunity: Adenosine, Lactate, Acidosis, Vascular Endothelial Growth Factor, Potassium Ions, and Phosphatidylserine
Abstract
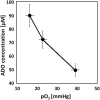
In this minireview, we aim to highlight key factors of the tumor microenvironment, including adenosine, lactate, acidosis, vascular endothelial growth factor, phosphatidylserine, high extracellular K+ levels, and tumor hypoxia with respect to antitumor immune functions. Most solid tumors have an immature chaotic microvasculature that results in tumor hypoxia. Hypoxia is a key determinant of tumor aggressiveness and therapy resistance and hypoxia-related gene products can thwart antitumor immune responses.
Keywords: acidosis; adenosine; antitumor immunity; lactate; phosphatidylserine; potassium ions; tumor hypoxia; vascular endothelial growth factor.
Figures

References
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res (1989) 49:6449–65. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

