Optimization of Phenotyping Assays for the Model Monocot Setaria viridis
- PMID: 29312412
- PMCID: PMC5743732
- DOI: 10.3389/fpls.2017.02172
Optimization of Phenotyping Assays for the Model Monocot Setaria viridis
Abstract
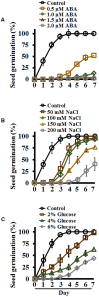
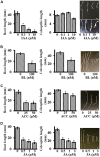
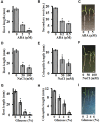
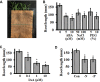
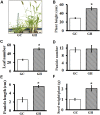
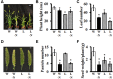
Setaria viridis (green foxtail) is an important model plant for the study of C4 photosynthesis in panicoid grasses, and is fast emerging as a system of choice for the study of plant development, domestication, abiotic stress responses and evolution. Basic research findings in Setaria are expected to advance research not only in this species and its close relative S. italica (foxtail millet), but also in other panicoid grasses, many of which are important food or bioenergy crops. Here we report on the standardization of multiple growth and development assays for S. viridis under controlled conditions, and in response to several phytohormones and abiotic stresses. We optimized these assays at three different stages of the plant's life: seed germination and post-germination growth using agar plate-based assays, early seedling growth and development using germination pouch-based assays, and adult plant growth and development under environmentally controlled growth chambers and greenhouses. These assays will be useful for the community to perform large scale phenotyping analyses, mutant screens, comparative physiological analysis, and functional characterization of novel genes of Setaria or other related agricultural crops. Precise description of various growth conditions, effective treatment conditions and description of the resultant phenotypes will help expand the use of S. viridis as an effective model system.
Keywords: Setaria viridis; abiotic stress; phenotyping; phytohormones; seed germination; seedling growth and development.
Figures



References
-
- Amini V., Zaefarian F., Rezvani M. (2015a). Interspecific variations in seed germination and seedling emergence of three Setaria species. Braz. J. Bot. 38 539–545. 10.1007/s40415-015-0158-6 - DOI
-
- Amini V., Zaefarian F., Rezvani M. (2015b). Effect of pre-chilling and environmental factors on breaking seed dormancy and germination of three foxtail species. Acta Agric. Slov. 105 269–278. 10.14720/aas.2015.105.2.10 - DOI
-
- Balemi T., Negisho K. (2012). Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J. Soil Sci. Plant Nutr. 12 547–561. 10.4067/S0718-95162012005000015 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

