Post-translational modifications of high mobility group box 1 and cancer
- PMID: 29312476
- PMCID: PMC5752874
Post-translational modifications of high mobility group box 1 and cancer
Abstract
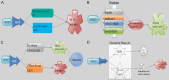
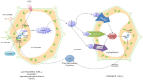
Post-translational modifications (PTMs) of High mobility group box 1 (HMGB1) have not been investigated as extensively as those of other HMG proteins but accumulating evidence has shown the remarkable biological significances induced by the post-translational: acetylation, methylation and phosphorylation, oxidation, glycosylation and ADP-ribosylation of the HMGB1 to modulate its interactions with DNA and other proteins. Although HMGB1 is localized in the nucleus in almost all cells at baseline, it can be rapidly mobilized to other sites within the cell, including the cytoplasm and mitochondria, as well as into the extracellular; hence there is an increasing interest by researches into the complex relationship between the PTMs of HMGB1 protein and its diverse biological activities. The PTMs of HMGB1 could also have effects on gene expression following changes in its DNA-binding properties and in extracellular environment displays immunological activity and could serve as a potential target for new therapy. Our reviewed identifies covalent modifications of HMGB1, and highlighted how these PTMs affect the functions of HMGB1 protein in a variety of cellular and extra cellular processes as well as diseases and therapy.
Keywords: HMGB1; autoimmune disease; cancer; modification; post-translational.
Conflict of interest statement
None.
Figures

References
-
- Richard SA, Xiang LH, Yun JX, Zhou SS, Jiang YY, Wang J, Su ZL, Xu HX. Carcinogenic and therapeutic role of high-mobility group Box 1 in cancer: is it a cancer facilitator, a cancer inhibitor or both? World Cancer Research Journal. 2017;4:e919.
-
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. - PubMed
-
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
