Secretory leukocyte protease inhibitor (SLPI) as a potential target for inhibiting metastasis of triple-negative breast cancers
- PMID: 29312532
- PMCID: PMC5752445
- DOI: 10.18632/oncotarget.22660
Secretory leukocyte protease inhibitor (SLPI) as a potential target for inhibiting metastasis of triple-negative breast cancers
Abstract
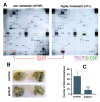
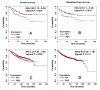
SLPI has been implicated in the progression and metastasis of certain cancers. However, the effects of SLPI seem to be tumor-specific and the mechanisms remain poorly defined. Here, we demonstrate that highly metastatic, triple-negative breast cancer (TNBC) 4T1 cells secreted more SLPI compared to their non-metastatic counterparts. Furthermore, SLPI secretion directly correlated with spontaneous lung metastasis from 4T1 tumors orthotopically implanted in mice. Consistent with our experimental results, we also found that higher SLPI expression levels correlate with worse clinical outcome in basal/TNBC patients. Using high-throughput screening we identified a novel compound, C74, which significantly inhibits SLPI secretion. C74 administration in our mouse model slows the growth of primary 4T1 tumors and inhibits their dissemination to the lung. We also discovered that SLPI physically interacts with the retinoblastoma tumor suppressor protein (Rb) and releases FoxM1 from the Rb-FoxM1 complex, which may activate FoxM1 target genes involved in breast cancer metastasis.
Keywords: FoxM1; SLPI; anti-metastatic compound; triple-negative breast cancer.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

