Targeting of CD122 enhances antitumor immunity by altering the tumor immune environment
- PMID: 29312597
- PMCID: PMC5752510
- DOI: 10.18632/oncotarget.22642
Targeting of CD122 enhances antitumor immunity by altering the tumor immune environment
Abstract
Mounting evidence demonstrates that CD8+CD122+ T cells have suppressive properties with the capacity to inhibit T cell responses. Therefore, these cells are rational targets for cancer immunotherapy. Here, we demonstrate that CD122 monoclonal antibody (mAb; aCD122) therapy significantly suppressed tumor growth and improved long-term survival in tumor-bearing mice. This therapeutic effect correlated with enhanced polyfunctional, cytolytic intratumoral CD8+ T cells and a decrease in granulocytic myeloid-derived suppressor cells (G-MDSCs). In addition, aCD122 treatment synergized with a vaccine to augment vaccine-induced antigen (Ag)-specific CD8+ T cell responses, reject established tumors and generate memory T cells. Furthermore, aCD122 mAb synergized with an anti-GITR (aGITR) mAb to confer significant control of tumor growth. These results suggest CD122 might be a promising target for cancer immunotherapy, either as a single agent or in combination with other forms of immunotherapy.
Keywords: CD122; CD8 T cells; GITR; immunotherapy; vaccines.
Conflict of interest statement
CONFLICTS OF INTEREST No potential conflicts of interest were disclosed by the authors.
Figures

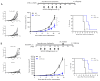




References
-
- Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+Cd4+ regulaotry T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

