Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas
- PMID: 29312603
- PMCID: PMC5752516
- DOI: 10.18632/oncotarget.22650
Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas
Abstract
Background: Recent studies have reported mutations in the telomerase reverse transcriptase promoter (TERTp) in meningiomas. We sought to determine the frequency, clonality and clinical significance of telomere gene alterations in a cohort of patients with progressive/higher-grade meningiomas.
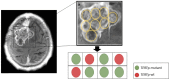
Methods: We characterized 64 temporally- and regionally-distinct specimens from 26 WHO grade III meningioma patients. On initial diagnoses, the meningiomas spanned all WHO grades (3 grade I, 13 grade II and 10 grade III). The tumor samples were screened for TERTp and ATRX/DAXX mutations, and TERT rearrangements. Additionally, TERTp was sequenced in a separate cohort of 19 patients with radiation-associated meningiomas. We examined the impact of mutational status on patients' progression and overall survival.
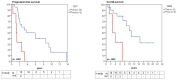
Results: Somatic TERTp mutations were detected in six patients (6/26 = 23%). Regional intratumoral heterogeneity in TERTp mutation status was noted. In 4 patients, TERTp mutations were detected in recurrent specimens but not in the available specimens of the first surgery. Additionally, a TERT gene fusion (LPCAT1-TERT) was found in one sample. In contrary, none of the investigated samples harbored an ATRX or DAXX mutation. In the cohort of radiation-induced meningiomas, TERTp mutation was detected in two patients (10.5%). Importantly, we found that patients with emergence of TERTp mutations had a substantially shorter OS than their TERTp wild-type counterparts (2.7 years, 95% CI 0.9 - 4.5 years versus 10.8 years, 95% CI 7.8 -12.8 years, p=0.003).
Conclusions: In progressive/higher-grade meningiomas,TERTp mutations are associated with poor survival, supporting a model in which selection of this alteration is a harbinger of aggressive tumor development. In addition, we observe spatial intratumoral heterogeneity of TERTp mutation status, consistent with this model of late emergence in tumor evolution. Thus, early detection of TERTp mutations may define patients with more aggressive meningiomas. Stratification for TERT alterations should be adopted in future clinical trials of progressive/higher-grade meningiomas.
Keywords: fusion; heterogeneity; meningioma; rearrangements; telomere.
Conflict of interest statement
CONFLICTS OF INTEREST All authors have no conflicts of interest to report with regard to this manuscript.
Figures
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

