Cationic Antimicrobial Peptides Inactivate Shiga Toxin-Encoding Bacteriophages
- PMID: 29312928
- PMCID: PMC5742231
- DOI: 10.3389/fchem.2017.00122
Cationic Antimicrobial Peptides Inactivate Shiga Toxin-Encoding Bacteriophages
Abstract
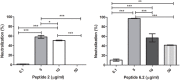
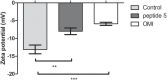
Shiga toxin (Stx) is the principal virulence factor during Shiga toxin-producing Escherichia coli (STEC) infections. We have previously reported the inactivation of bacteriophage encoding Stx after treatment with chitosan, a linear polysaccharide polymer with cationic properties. Cationic antimicrobial peptides (cAMPs) are short linear aminoacidic sequences, with a positive net charge, which display bactericidal or bacteriostatic activity against a wide range of bacterial species. They are promising novel antibiotics since they have shown bactericidal effects against multiresistant bacteria. To evaluate whether cationic properties are responsible for bacteriophage inactivation, we tested seven cationic peptides with proven antimicrobial activity as anti-bacteriophage agents, and one random sequence cationic peptide with no antimicrobial activity as a control. We observed bacteriophage inactivation after incubation with five cAMPs, but no inactivating activity was observed with the random sequence cationic peptide or with the non-alpha helical cAMP Omiganan. Finally, to confirm peptide-bacteriophage interaction, zeta potential was analyzed by following changes on bacteriophage surface charges after peptide incubation. According to our results we could propose that: (1) direct interaction of peptides with phage is a necessary step for bacteriophage inactivation, (2) cationic properties are necessary but not sufficient for bacteriophage inactivation, and (3) inactivation by cationic peptides could be sequence (or structure) specific. Overall our data suggest that these peptides could be considered a new family of molecules potentially useful to decrease bacteriophage replication and Stx expression.
Keywords: Escherichia coli O157; anti-infective agents; antimicrobial peptides; bacteriophages (phages); hemolytic uremic syndrome (HUS).
Figures



References
-
- Hollmann A., Martínez M., Noguera M. E., Augusto M. T., Disalvo A., Santos N. C., et al. (2016). Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids Surf. B Biointerfaces 141, 528–536. 10.1016/j.colsurfb.2016.02.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

