The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome
- PMID: 29312942
- PMCID: PMC5735370
- DOI: 10.3389/fmed.2017.00224
The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome
Abstract
Background: The increased incidence and geographic expansion of Lyme disease has made it the most common vector-borne infection in North America. Posttreatment Lyme disease syndrome (PTLDS) represents a subset of patients who remain ill following standard antibiotic therapy for Lyme disease. The spectrum of symptoms and their impact on quality of life remain largely unexplored among patients with well-documented PTLDS.
Objective: To characterize a case series of patients with well-documented PTLDS compared to a sample of healthy controls.
Methods: Sixty-one participants met the proposed case definition for PTLDS. Twenty-six healthy controls had neither a clinical history of Lyme disease nor current antibodies to Borrelia burgdorferi. Participants with PTLDS and controls were evaluated by physical exam, clinical laboratory testing, standardized questionnaires, and a 36-item current symptom list.
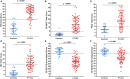
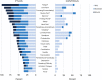
Results: Compared to controls, participants with PTLDS reported significantly greater fatigue, pain, sleep disturbance, and depression (Fatigue Severity Scale: 50.0 ± 10.6 vs. 19.8 ± 8.6; Short-Form McGill Pain Questionnaire: 13.7 ± 8.3 vs. 0.8 ± 1.9; Pittsburgh Sleep Quality Index: 10.1 ± 4.7 vs. 4.1 ± 2.1; Beck Depression Inventory-II: 15.1 ± 7.7 vs. 2.2 ± 3.2; p < 0.001 for each), and significantly lower quality of life (SF-36 Physical Component Score: 33.9 ± 9.7 vs. 55.1 ± 6.2; Mental Component Score: 42.9 ± 10.1 vs. 54.2 ± 5.4; p < 0.001 for each). Nineteen non-PTLDS-defining symptoms were found to be significantly more severe among participants with PTLDS than controls, including sleep difficultly and visual complaints. Initial delayed or misdiagnosis was characterized in 59.0% of participants with PTLDS, and 32.2% had abnormal vibratory sense.
Conclusion: Although physical exam and clinical laboratory tests showed few objective abnormalities, standardized symptom questionnaires revealed that patients with PTLDS are highly and clinically significantly symptomatic, with poor health-related quality of life. PTLDS patients exhibited levels of fatigue, musculoskeletal pain, sleep disturbance, and depression which were both clinically relevant and statistically significantly higher than controls. Our study shows that PTLDS can be successfully identified using a systematic approach to diagnosis and symptom measurement. As the prevalence of PTLDS continues to rise, there will be an increased need for physician education to more effectively identify and manage PTLDS as part of integrated patient care.
Keywords: Lyme disease; case series; posttreatment Lyme disease syndrome; quality of life; signs and symptoms.
Figures
References
-
- Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, et al. Summary of notifiable diseases – United States, 2012. MMWR Morb Mortal Wkly Rep (2014) 61(53):1–121. - PubMed
-
- Centers for Disease Control and Prevention. Lyme Disease Data and Statistics (2017). Available from: http://www.cdc.gov/lyme/stats/index.html
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

