Age-Associated Decline in Dendritic Cell Function and the Impact of Mediterranean Diet Intervention in Elderly Subjects
- PMID: 29312949
- PMCID: PMC5742184
- DOI: 10.3389/fnut.2017.00065
Age-Associated Decline in Dendritic Cell Function and the Impact of Mediterranean Diet Intervention in Elderly Subjects
Abstract
Introduction: Aging is accompanied by increased susceptibility to infection and age-associated chronic diseases. It is also associated with reduced vaccine responses, which is often attributed to immunosenescence and the functional decline of the immune system. Immunosenescence is characterized by a chronic, low-grade, inflammatory state termed inflammaging. Habitants of Mediterranean (MED) regions maintain good health into old age; often attributed to MED diets.
Hypothesis: Adoption of a MED-diet by elderly subjects, in Norfolk (UK), may improve immune responses of these individuals and in particular, dendritic cell (DC) function.
Experimental approach: A total of 120 elderly subjects (65-79 years old) recruited onto the Nu-AGE study, a multicenter European dietary study specifically addressing the needs of the elderly, across five countries, and were randomized to the control or MED-diet groups, for one year. Blood samples were taken pre- and post-intervention for DC analysis and were compared with each other, and to samples obtained from 45 young (18-40 years old) subjects. MED-diet compliance was assessed using high performance liquid chromatography-with tandem mass spectrometry analysis of urine samples. Immune cell and DC subset numbers and concentrations of secreted proteins were determined by flow cytometric analysis.
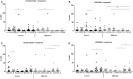
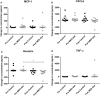
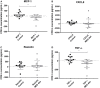
Results: As expected, reduced myeloid DC numbers were observed in blood samples from elderly subjects compared with young. The elevated secretion of the adipokine, resistin, after ex vivo stimulation of peripheral blood mononuclear cells from elderly subjects, was significantly reduced after MED-diet intervention.
Conclusion: This study provides further evidence of numerical and functional effects of aging on DCs. The MED-diet showed potential to impact on the aging immune cells investigated and could provide an economical approach to address problems associated with our aging population.
Keywords: Mediterranean diet; aging; cytokines; dendritic cells; resistin.
Figures



References
-
- Pawelec G. Does the human immune system ever really become “senescent”? [version 1; referees: 5 approved] F1000Res (2017) 6(F1000 Faculty Rev):1323. 10.12688/f1000research.11297.1 - DOI
-
- Dragsbæk K, Neergaard JS, Laursen JM, Hansen HB, Christiansen C, Beck-Nielsen H, et al. Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine (Baltimore) (2016) 95(36):e4806. 10.1097/md.0000000000004806 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

