Nanoparticles in the clinic
- PMID: 29313004
- PMCID: PMC5689513
- DOI: 10.1002/btm2.10003
Nanoparticles in the clinic
Abstract
Nanoparticle/microparticle-based drug delivery systems for systemic (i.e., intravenous) applications have significant advantages over their nonformulated and free drug counterparts. For example, nanoparticle systems are capable of delivering therapeutics and treating areas of the body that other delivery systems cannot reach. As such, nanoparticle drug delivery and imaging systems are one of the most investigated systems in preclinical and clinical settings. Here, we will highlight the diversity of nanoparticle types, the key advantages these systems have over their free drug counterparts, and discuss their overall potential in influencing clinical care. In particular, we will focus on current clinical trials for nanoparticle formulations that have yet to be clinically approved. Additional emphasis will be on clinically approved nanoparticle systems, both for their currently approved indications and their use in active clinical trials. Finally, we will discuss many of the often overlooked biological, technological, and study design challenges that impact the clinical success of nanoparticle delivery systems.
Keywords: clinic; clinical translation; clinical trials; drug delivery; nanomedicine; nanoparticles; translational medicine.
Figures

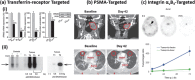
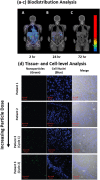

References
-
- Svenson S. Clinical translation of nanomedicines. Curr Opin Solid State Mater Sci. 2012;16(6):287–294.
-
- Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24(10):1211–1217. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

