Evolving trends in mAb production processes
- PMID: 29313024
- PMCID: PMC5689530
- DOI: 10.1002/btm2.10061
Evolving trends in mAb production processes
Abstract
Monoclonal antibodies (mAbs) have established themselves as the leading biopharmaceutical therapeutic modality. The establishment of robust manufacturing platforms are key for antibody drug discovery efforts to seamlessly translate into clinical and commercial successes. Several drivers are influencing the design of mAb manufacturing processes. The advent of biosimilars is driving a desire to achieve lower cost of goods and globalize biologics manufacturing. High titers are now routinely achieved for mAbs in mammalian cell culture. These drivers have resulted in significant evolution in process platform approaches. Additionally, several new trends in bioprocessing have arisen in keeping with these needs. These include the consideration of alternative expression systems, continuous biomanufacturing and non-chromatographic separation formats. This paper discusses these drivers in the context of the kinds of changes they are driving in mAb production processes.
Keywords: Fc fusion proteins; biosimilars; bispecific antibodies; continuous bioprocessing; high titer cell culture; monoclonal antibodies; next generation monoclonal antibodies; nonchromatographic separations; platform process; process development.
Figures

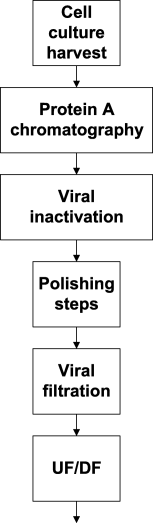


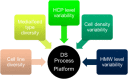
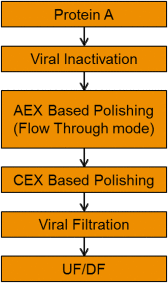
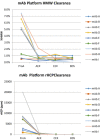
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

