Histone H3 lysine 4 monomethylation modulates long-range chromatin interactions at enhancers
- PMID: 29313530
- PMCID: PMC5799818
- DOI: 10.1038/cr.2018.1
Histone H3 lysine 4 monomethylation modulates long-range chromatin interactions at enhancers
Erratum in
-
Histone H3 lysine 4 monomethylation modulates long-range chromatin interactions at enhancers.Cell Res. 2018 Mar;28(3):387. doi: 10.1038/cr.2018.18. Cell Res. 2018. PMID: 29497152 Free PMC article.
Abstract
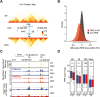
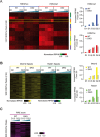
Long-range chromatin interactions between enhancers and promoters are essential for transcription of many developmentally controlled genes in mammals and other metazoans. Currently, the exact mechanisms that connect distal enhancers to their specific target promoters remain to be fully elucidated. Here, we show that the enhancer-specific histone H3 lysine 4 monomethylation (H3K4me1) and the histone methyltransferases MLL3 and MLL4 (MLL3/4) play an active role in this process. We demonstrate that in differentiating mouse embryonic stem cells, MLL3/4-dependent deposition of H3K4me1 at enhancers correlates with increased levels of chromatin interactions, whereas loss of this histone modification leads to reduced levels of chromatin interactions and defects in gene activation during differentiation. H3K4me1 facilitates recruitment of the Cohesin complex, a known regulator of chromatin organization, to chromatin in vitro and in vivo, providing a potential mechanism for MLL3/4 to promote chromatin interactions between enhancers and promoters. Taken together, our results support a role for MLL3/4-dependent H3K4me1 in orchestrating long-range chromatin interactions at enhancers in mammalian cells.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

