Layer-specific excitation/inhibition balances during neuronal synchronization in the visual cortex
- PMID: 29313982
- PMCID: PMC5924838
- DOI: 10.1113/JP274986
Layer-specific excitation/inhibition balances during neuronal synchronization in the visual cortex
Abstract
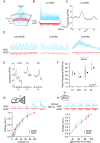
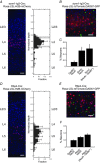
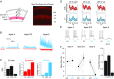
Key points: Understanding the balance between synaptic excitation and inhibition in cortical circuits in the brain, and how this contributes to cortical rhythms, is fundamental to explaining information processing in the cortex. This study used cortical layer-specific optogenetic activation in mouse cortex to show that excitatory neurons in any cortical layer can drive powerful gamma rhythms, while inhibition balances excitation. The net impact of this is to keep activity within each layer in check, but simultaneously to promote the propagation of activity to downstream layers. The data show that rhythm-generating circuits exist in all principle layers of the cortex, and provide layer-specific balances of excitation and inhibition that affect the flow of information across the layers.
Abstract: Rhythmic activity can synchronize neural ensembles within and across cortical layers. While gamma band rhythmicity has been observed in all layers, the laminar sources and functional impacts of neuronal synchronization in the cortex remain incompletely understood. Here, layer-specific optogenetic stimulation demonstrates that populations of excitatory neurons in any cortical layer of the mouse's primary visual cortex are sufficient to powerfully entrain neuronal oscillations in the gamma band. Within each layer, inhibition balances excitation and keeps activity in check. Across layers, translaminar output overcomes inhibition and drives downstream firing. These data establish that rhythm-generating circuits exist in all principle layers of the cortex, but provide layer-specific balances of excitation and inhibition that may dynamically shape the flow of information through cortical circuits. These data might help explain how excitation/inhibition (E/I) balances across cortical layers shape information processing, and shed light on the diverse nature and functional impacts of cortical gamma rhythms.
Keywords: E/I balance; V1; balance of excitation and inhibition; cortical circuits; cortical layers; gamma oscillations; optogenetics; visual cortex.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures




Comment in
-
The songs of silence: cortical suppression and synchronization by layer-specific activation.J Physiol. 2018 May 1;596(9):1541-1542. doi: 10.1113/JP275797. Epub 2018 Mar 23. J Physiol. 2018. PMID: 29512157 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

