Inhibition of CaMKK2 Enhances Fracture Healing by Stimulating Indian Hedgehog Signaling and Accelerating Endochondral Ossification
- PMID: 29314250
- PMCID: PMC6549722
- DOI: 10.1002/jbmr.3379
Inhibition of CaMKK2 Enhances Fracture Healing by Stimulating Indian Hedgehog Signaling and Accelerating Endochondral Ossification
Abstract
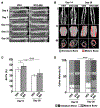
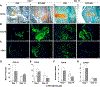
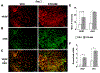
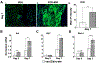
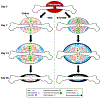
Approximately 10% of all bone fractures do not heal, resulting in patient morbidity and healthcare costs. However, no pharmacological treatments are currently available to promote efficient bone healing. Inhibition of Ca2+ /calmodulin (CaM)-dependent protein kinase kinase 2 (CaMKK2) reverses age-associated loss of trabecular and cortical bone volume and strength in mice. In the current study, we investigated the role of CaMKK2 in bone fracture healing and show that its pharmacological inhibition using STO-609 accelerates early cellular and molecular events associated with endochondral ossification, resulting in a more rapid and efficient healing of the fracture. Within 7 days postfracture, treatment with STO-609 resulted in enhanced Indian hedgehog signaling, paired-related homeobox (PRX1)-positive mesenchymal stem cell (MSC) recruitment, and chondrocyte differentiation and hypertrophy, along with elevated expression of osterix, vascular endothelial growth factor, and type 1 collagen at the fracture callus. Early deposition of primary bone by osteoblasts resulted in STO-609-treated mice possessing significantly higher callus bone volume by 14 days following fracture. Subsequent rapid maturation of the bone matrix bestowed fractured bones in STO-609-treated animals with significantly higher torsional strength and stiffness by 28 days postinjury, indicating accelerated healing of the fracture. Previous studies indicate that fixed and closed femoral fractures in the mice take 35 days to fully heal without treatment. Therefore, our data suggest that STO-609 potentiates a 20% acceleration of the bone healing process. Moreover, inhibiting CaMKK2 also imparted higher mechanical strength and stiffness at the contralateral cortical bone within 4 weeks of treatment. Taken together, the data presented here underscore the therapeutic potential of targeting CaMKK2 to promote efficacious and rapid healing of bone fractures and as a mechanism to strengthen normal bones. © 2018 American Society for Bone and Mineral Research.
Keywords: ANABOLICS; ANIMAL MODELS; INJURY/FRACTURE HEALING; ORTHOPAEDICS; PRECLINICAL STUDIES; THERAPEUTICS.
© 2018 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosures
All authors of this manuscript state that they have no conflict of interest. The authors further state that there are no restrictions on full access for all authors to all raw data, statistical analyses and material used in the study reported in this manuscript.
Figures

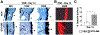
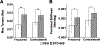
References
-
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. March 2007;22(3):465–75. - PubMed
-
- Covey DC, Aaron RK, Born CT, Calhoun JH, Einhorn TA, Hayda RA, et al. Orthopaedic war injuries: from combat casualty care to definitive treatment: a current review of clinical advances, basic science, and research opportunities. Instr Course Lect. 2008;57:65–86. Epub 2008/04/11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

