Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance
- PMID: 29314558
- PMCID: PMC5910259
- DOI: 10.1111/ajt.14645
Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance
Abstract
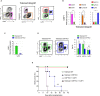
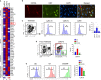
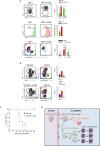
The colony-stimulating factor 1 (CSF1) regulates the differentiation and function of tissue macrophages and determines the outcome of the immune response. The molecular mechanisms behind CSF1-mediated macrophage development remain to be elucidated. Here we demonstrate that neutrophil-derived CSF1 controls macrophage polarization and proliferation, which is necessary for the induction of tolerance. Inhibiting neutrophil production of CSF1 or preventing macrophage proliferation, using targeted nanoparticles loaded with the cell cycle inhibitor simvastatin, abrogates the induction of tolerance. These results provide new mechanistic insights into the developmental requirements of tolerogenic macrophages and identify CSF1 producing neutrophils as critical regulators of the immunological response.
Keywords: basic (laboratory) research/science; immunobiology; macrophage/monocyte biology: differentiation/maturation; tolerance: mechanisms.
© 2018 The Authors. American Journal of Transplantation published by Wiley Periodicals, Inc. on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
References
-
- Poulter LW, Bradley NJ, Turk JL. The role of macrophages in skin allograft rejection. I. Histochemical studies during first‐set rejection. Transplantation. 1971;12(1):40‐44. - PubMed
-
- Wyburn KR, Jose MD, Wu H, Atkins RC, Chadban SJ. The role of macrophages in allograft rejection. Transplantation. 2005;80(12):1641‐1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

