Investigating Protein-Ligand Interactions by Solution Nuclear Magnetic Resonance Spectroscopy
- PMID: 29314603
- PMCID: PMC5915746
- DOI: 10.1002/cphc.201701253
Investigating Protein-Ligand Interactions by Solution Nuclear Magnetic Resonance Spectroscopy
Abstract
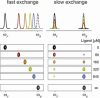
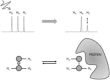
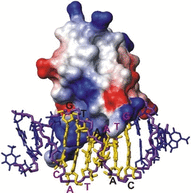
Protein-ligand interactions are of fundamental importance in almost all processes in living organisms. The ligands comprise small molecules, drugs or biological macromolecules and their interaction strength varies over several orders of magnitude. Solution NMR spectroscopy offers a large repertoire of techniques to study such complexes. Here, we give an overview of the different NMR approaches available. The information they provide ranges from the simple information about the presence of binding or epitope mapping to the complete 3 D structure of the complex. NMR spectroscopy is particularly useful for the study of weak interactions and for the screening of binding ligands with atomic resolution.
Keywords: chemical shift mapping; nuclear Overhauser effect; nuclear magnetic resonance; protein-ligand interactions; saturation-transfer difference.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

