Transcriptome profiling of peripheral blood immune cell populations in multiple sclerosis patients before and during treatment with a sphingosine-1-phosphate receptor modulator
- PMID: 29314605
- PMCID: PMC6490155
- DOI: 10.1111/cns.12793
Transcriptome profiling of peripheral blood immune cell populations in multiple sclerosis patients before and during treatment with a sphingosine-1-phosphate receptor modulator
Abstract
Aims: Fingolimod is a sphingosine-1-phosphate (S1P) receptor modulator approved for the treatment of the relapsing form of multiple sclerosis (MS). It prevents the egress of lymphocyte subpopulations from lymphoid tissues into the circulation. Here, we explored the broad effects of fingolimod on gene expression in different immune cell subsets.
Methods: Utilizing 150 high-resolution microarrays from Affymetrix, we obtained the transcriptome profiles of 5 cell populations, which were separated from the peripheral blood of MS patients prior to and following oral administration of fingolimod.
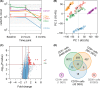
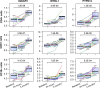
Results: After 3 months of treatment, significant transcriptome shifts were seen in CD4+ and CD8+ cells, which is mainly attributable to the selective homing of naive T cells and central memory T cells. Although the number of B cells was greatly reduced in the blood of fingolimod-treated MS patients, the analysis of differential expression in CD19+ cells identified only a small set of 42 genes, which indicated a slightly higher frequency of transitional B cells. The transcriptome signatures of CD14+ monocytes and CD56+ natural killer cells were not affected.
Conclusion: Our study corroborates changes in the composition of circulating immune cells in response to fingolimod and delineates the respective implications at the RNA level. Our data may be valuable for comparing the effects of novel S1P receptor modulating agents, which may be a therapeutic option for patients with secondary progressive MS as well.
Keywords: CD19+ B cells; fingolimod therapy; peripheral blood; relapsing-remitting multiple sclerosis; transcriptome microarray analysis.
© 2018 John Wiley & Sons Ltd.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

