Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: Evidence of clinical utility and cost effectiveness
- PMID: 29314763
- PMCID: PMC5902395
- DOI: 10.1002/mgg3.355
Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: Evidence of clinical utility and cost effectiveness
Abstract
Background: Epileptic encephalopathies are a devastating group of neurological conditions in which etiological diagnosis can alter management and clinical outcome. Exome sequencing and gene panel testing can improve diagnostic yield but there is no cost-effectiveness analysis of their use or consensus on how to best integrate these tests into clinical diagnostic pathways.
Methods: We conducted a retrospective cost-effectiveness study comparing trio exome sequencing with a standard diagnostic approach, for a well-phenotyped cohort of 32 patients with epileptic encephalopathy, who remained undiagnosed after "first-tier" testing. Sensitivity analysis was included with a range of commercial exome and multigene panels.
Results: The diagnostic yield was higher for the exome sequencing (16/32; 50%) than the standard arm (2/32; 6.2%). The trio exome sequencing pathway was cost-effective compared to the standard diagnostic pathway with a cost saving of AU$5,236 (95% confidence intervals $2,482; $9,784) per additional diagnosis; the standard pathway cost approximately 10 times more per diagnosis. Sensitivity analysis demonstrated that the majority of commercial exome sequencing and multigene panels studied were also cost-effective. The clinical utility of all diagnoses was reported.
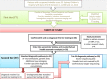
Conclusion: Our study supports the integration of exome sequencing and gene panel testing into the diagnostic pathway for epileptic encephalopathy, both in terms of cost effectiveness and clinical utility. We propose a diagnostic pathway that integrates initial rapid screening for treatable causes and comprehensive genomic screening. This study has important implications for health policy and public funding for epileptic encephalopathy and other neurological conditions.
Keywords: diagnosis; epilepsy; genomics; health economics.
© 2017 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Figures




References
-
- Amendola, L. M. , Dorschner, M. O. , Robertson, P. D. , Salama, J. S. , Hart, R. , Shirts, B. H. , … Jarvik, G. P. (2015). Actionable exomic incidental findings in 6503 participants: Challenges of variant classification. Genome Research, 25, 305–315. https://doi.org/10.1101/gr.183483.114 - DOI - PMC - PubMed
-
- Beghi, E. , Frigeni, B. , Beghi, M. , De Compadri, P. , & Garattini, L. (2005). A review of the costs of managing childhood epilepsy. Pharmacoeconomics, 23, 27–45. https://doi.org/10.2165/00019053-200523010-00003 - DOI - PubMed
-
- Berg, A. T. , Berkovic, S. F. , Brodie, M. J. , Buchhalter, J. , Cross, J. H. , van Emde Boas, W. , … Scheffer, I. E. (2010). Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia, 51, 676–685. https://doi.org/10.1111/j.1528-1167.2010.02522.x - DOI - PubMed
-
- Berg, A. T. , Coryell, J. , Saneto, R. P. , Grinspan, Z. M. , Alexander, J. J. , Kekis, M. , … Koh, S. (2017). Early‐life epilepsies and the emerging role of genetic testing. JAMA Pediatrics, 171, 863–871. https://doi.org/10.1001/jamapediatrics.2017.1743 - DOI - PMC - PubMed
-
- Berkovic, S. F. (2015). Genetics of epilepsy in clinical practice. Epilepsy Currents, 15, 192–196. https://doi.org/10.5698/1535-7511-15.4.192 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

