Bacterial-derived Neutrophilic Inflammation Drives Lung Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease
- PMID: 29314863
- PMCID: PMC6002662
- DOI: 10.1165/rcmb.2017-0329OC
Bacterial-derived Neutrophilic Inflammation Drives Lung Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease
Abstract
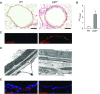
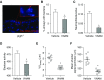
Loss of secretory IgA is common in the small airways of patients with chronic obstructive pulmonary disease and may contribute to disease pathogenesis. Using mice that lack secretory IgA in the airways due to genetic deficiency of polymeric Ig receptor (pIgR-/- mice), we investigated the role of neutrophils in driving the fibrotic small airway wall remodeling and emphysema that develops spontaneously in these mice. By flow cytometry, we found an increase in the percentage of neutrophils among CD45+ cells in the lungs, as well as an increase in total neutrophils, in pIgR-/- mice compared with wild-type controls. This increase in neutrophils in pIgR-/- mice was associated with elastin degradation in the alveolar compartment and around small airways, along with increased collagen deposition in small airway walls. Neutrophil depletion using anti-Ly6G antibodies or treatment with broad-spectrum antibiotics inhibited development of both emphysema and small airway remodeling, suggesting that airway bacteria provide the stimulus for deleterious neutrophilic inflammation in this model. Exogenous bacterial challenge using lysates prepared from pathogenic and nonpathogenic bacteria worsened neutrophilic inflammation and lung remodeling in pIgR-/- mice. This phenotype was abrogated by antiinflammatory therapy with roflumilast. Together, these studies support the concept that disruption of the mucosal immune barrier in small airways contributes to chronic obstructive pulmonary disease progression by allowing bacteria to stimulate chronic neutrophilic inflammation, which, in turn, drives progressive airway wall fibrosis and emphysematous changes in the lung parenchyma.
Keywords: chronic obstructive pulmonary disease; emphysema; mucosal immunity; polymeric Ig receptor; secretory IgA.
Figures





Comment in
-
Does Compromised Immune Exclusion Drive Inflammatory Processes in Chronic Obstructive Pulmonary Disease?Am J Respir Cell Mol Biol. 2018 Jun;58(6):671-672. doi: 10.1165/rcmb.2018-0039ED. Am J Respir Cell Mol Biol. 2018. PMID: 29856258 No abstract available.
References
-
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. - PubMed
-
- Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis. 1983;127:474–477. - PubMed
-
- Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koëter GH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax. 2000;55:12–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

