Surface Decoration of ZnWO₄ Nanorods with Cu₂O Nanoparticles to Build Heterostructure with Enhanced Photocatalysis
- PMID: 29315264
- PMCID: PMC5791120
- DOI: 10.3390/nano8010033
Surface Decoration of ZnWO₄ Nanorods with Cu₂O Nanoparticles to Build Heterostructure with Enhanced Photocatalysis
Abstract
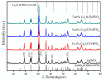
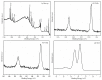
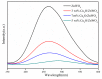
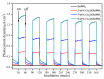
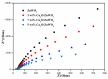
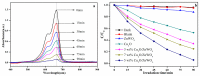
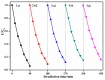
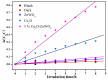
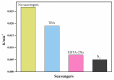
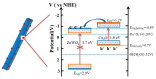
The surface of ZnWO₄ nanorods was decorated with Cu₂O nanoparticles (Cu₂O/ZnWO₄) prepared through a precipitation method. The Cu₂O nanoparticles were tightly deposited on the ZnWO₄ surface and had average diameters of 20 nm. The nanoparticles not only promoted the absorption and utilization of visible light but also facilitated the separation of photogenerated charge carriers. This brought an improvement of the photocatalytic activity. The 5 wt % Cu₂O/ZnWO₄ photocatalyst displayed the highest degrade efficiency for methylene blue (MB) degradation under visible light, which was 7.8 and 2 times higher than pure ZnWO₄ and Cu₂O, respectively. Meanwhile, the Cu₂O/ZnWO₄ composite photocatalyst was able to go through phenol degradation under visible light. The results of photoluminescence (PL), photocurrent, and electrochemical impedance spectra (EIS) measurements were consistent and prove the rapid separation of charge, which originated from the match level structure and the close contact with the interface. The radical and hole trapping experiments were carried out to detect the main active substances in the photodegradation process. The holes and ·O₂- radicals were predicted to dominate the photocatalytic process. Based on the characterization analysis and experiment results, a possible photocatalytic mechanism for enhancing photocatalytic activity was proposed.
Keywords: ZnWO4; degradation; nanoparticles; photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Patnaik S., Martha S., Parida K.M. An overview of the structural, textural and morphological modulations of g-C3N4 towards photocatalytic hydrogen production. RSC Adv. 2016;6:46929–46951. doi: 10.1039/C5RA26702A. - DOI
-
- Sun L.M., Zhao X., Cheng X.F., Sun H.G., Li Y.L., Li P., Fan W.L. Evaluating the C, N, and F Pairwise Codoping Effect on the Enhanced Photoactivity of ZnWO4: The Charge Compensation Mechanism in Donor Acceptor Pairs. J. Phys. Chem. C. 2011;115:15516–15524. doi: 10.1021/jp204324n. - DOI
-
- Yu C.L., Yu J.C. Sonochemical fabrication, characterization and photocatalytic properties of Ag/ZnWO4 nanorod catalyst. Mater. Sci. Eng. B. 2009;164:16–22. doi: 10.1016/j.mseb.2009.06.008. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

