Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective
- PMID: 29315504
- PMCID: PMC6032820
- DOI: 10.1002/cpt.1013
Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective
Abstract
This work provides a perspective on the qualification and verification of physiologically based pharmacokinetic (PBPK) platforms/models intended for regulatory submission based on the collective experience of the Simcyp Consortium members. Examples of regulatory submission of PBPK analyses across various intended applications are presented and discussed. European Medicines Agency (EMA) and US Food and Drug Administration (FDA) recent draft guidelines regarding PBPK analyses and reporting are encouraging, and to advance the use and acceptability of PBPK analyses, more clarity and flexibility are warranted.
© 2018, The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

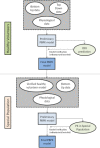
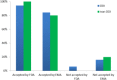
References
-
- Jones, H.M. et al Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin. Pharmacol. Ther. 97, 247–262 (2015). - PubMed
-
- Luzon, E. , Blake, K. , Cole, S. , Nordmark, A. , Versantvoort, C. & Berglund, E.G. Physiologically based pharmacokinetic modeling in regulatory decision‐making at the European Medicines Agency. Clin. Pharmacol. Ther. DOI: 10.1002/cpt.1539 (2016) [Epub ahead of print]. - DOI - PubMed
-
- United States Food and Drug Administration. Guidance for Industry: physiologically Based Pharmacokinetic Analyses—Format and Content. December, 2016. <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat...>.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

