Bidirectional variation in glutamate efflux in the medial prefrontal cortex induced by selective positive and negative allosteric mGluR5 modulators
- PMID: 29315577
- PMCID: PMC5972455
- DOI: 10.1111/jnc.14290
Bidirectional variation in glutamate efflux in the medial prefrontal cortex induced by selective positive and negative allosteric mGluR5 modulators
Abstract
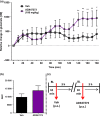
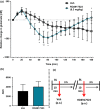
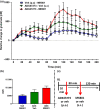
Dysregulation of prefrontal cortical glutamatergic signalling via NMDA receptor hypofunction has been implicated in cognitive dysfunction and impaired inhibitory control in such neuropsychiatric disorders as schizophrenia, attention-deficit hyperactivity disorder and drug addiction. Although NMDA receptors functionally interact with metabotropic glutamate receptor 5 (mGluR5), the consequence of this interaction for glutamate release in the prefrontal cortex (PFC) remains unknown. We therefore investigated the effects of positive and negative allosteric mGluR5 modulation on changes in extracellular glutamate efflux in the medial PFC (mPFC) induced by systemic administration of the non-competitive NMDA receptor antagonist dizocilpine (or MK801) in rats. Extracellular glutamate efflux was measured following systemic administration of the positive allosteric mGluR5 modulator [S-(4-Fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]-oxadiazol-5-yl]-piperidin-1-yl}-methanone] (ADX47273; 100 mg/kg, p.o.) and negative allosteric mGluR5 modulator [2-chloro-4-{[1-(4-fluorophenyl)-2,5-dimethyl-1H-imidazol-4-yl]ethynyl}pyridine] (RO4917523; 0.3 mg/kg, p.o.), using a wireless glutamate biosensor in awake, freely moving rats. The effect of MK801 (0.03-0.06 mg/kg, s.c.) on mPFC glutamate efflux was also investigated in addition to the effects of MK801 (0.03 mg/kg, s.c.) following ADX47273 (100 mg/kg, p.o.) pre-treatment. ADX47273 produced a sustained increase in glutamate efflux and increased the effect of NMDA receptor antagonism on glutamate efflux in the mPFC. In contrast, negative allosteric mGluR5 modulation with RO4917523 decreased glutamate efflux in the mPFC. These findings indicate that positive and negative allosteric mGluR5 modulators produce long lasting and opposing actions on extracellular glutamate efflux in the mPFC. Positive and negative allosteric modulators of mGluR5 may therefore be viable therapeutic agents to correct abnormalities in glutamatergic signalling present in a range of neuropsychiatric disorders.
Keywords: NMDA receptors; glutamate; impulsivity; mGluR5; prefrontal cortex.
© 2018 International Society for Neurochemistry.
Figures



References
-
- Abekawa T., Honda M., Ito K. and Koyama T. (2003) Effects of NRA0045, a novel potent antagonist at dopamine D4, 5‐HT2A, and α1 adrenaline receptors, and NRA0160, a selective D4 receptor antagonist, on phencyclidine‐induced behavior and glutamate release in rats. Psychopharmacology 169, 247–256. - PubMed
-
- Adams B. W. and Moghaddam B. (2001) Effect of clozapine, haloperidol, or M100907 on phencyclidine‐activated glutamate efflux in the prefrontal cortex. Biol. Psychiatry 50, 750–757. - PubMed
-
- Agnoli L. and Carli M. (2012) Dorsal–striatal 5‐HT2A and 5‐HT2C receptors control impulsivity and perseverative responding in the 5‐choice serial reaction Time Task. Psychopharmacology 219, 633–645. - PubMed
-
- Alagarsamy S., Rouse S. T., Junge C., Hubert G. W., Gutman D., Smith Y. and Conn P. J. (2002) NMDA‐induced phosphorylation and regulation of mGluR5. Pharmacol. Biochem. Behav. 73, 299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

