Defining the phenotypic spectrum of SLC6A1 mutations
- PMID: 29315614
- PMCID: PMC5912688
- DOI: 10.1111/epi.13986
Defining the phenotypic spectrum of SLC6A1 mutations
Abstract
Objective: Pathogenic SLC6A1 variants were recently described in patients with myoclonic atonic epilepsy (MAE) and intellectual disability (ID). We set out to define the phenotypic spectrum in a larger cohort of SCL6A1-mutated patients.
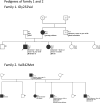
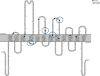
Methods: We collected 24 SLC6A1 probands and 6 affected family members. Four previously published cases were included for further electroclinical description. In total, we reviewed the electroclinical data of 34 subjects.
Results: Cognitive development was impaired in 33/34 (97%) subjects; 28/34 had mild to moderate ID, with language impairment being the most common feature. Epilepsy was diagnosed in 31/34 cases with mean onset at 3.7 years. Cognitive assessment before epilepsy onset was available in 24/31 subjects and was normal in 25% (6/24), and consistent with mild ID in 46% (11/24) or moderate ID in 17% (4/24). Two patients had speech delay only, and 1 had severe ID. After epilepsy onset, cognition deteriorated in 46% (11/24) of cases. The most common seizure types were absence, myoclonic, and atonic seizures. Sixteen cases fulfilled the diagnostic criteria for MAE. Seven further patients had different forms of generalized epilepsy and 2 had focal epilepsy. Twenty of 31 patients became seizure-free, with valproic acid being the most effective drug. There was no clear-cut correlation between seizure control and cognitive outcome. Electroencephalography (EEG) findings were available in 27/31 patients showing irregular bursts of diffuse 2.5-3.5 Hz spikes/polyspikes-and-slow waves in 25/31. Two patients developed an EEG pattern resembling electrical status epilepticus during sleep. Ataxia was observed in 7/34 cases. We describe 7 truncating and 18 missense variants, including 4 recurrent variants (Gly232Val, Ala288Val, Val342Met, and Gly362Arg).
Significance: Most patients carrying pathogenic SLC6A1 variants have an MAE phenotype with language delay and mild/moderate ID before epilepsy onset. However, ID alone or associated with focal epilepsy can also be observed.
Keywords: MAE; SLC6A1; epilepsy; epilepsy genetics.
Wiley Periodicals, Inc. © 2018 International League Against Epilepsy.
Conflict of interest statement
ST and KLH are employed by Ambry Genetics. CC was funded by the SNSF Early Postdoc fellowship. YW and IH were funded by DFG We4896/3-1; He5415/6-1. The remaining authors have no conflicts of interest to disclose. added. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
-
- Madsen KK, Hansen GH, Danielsen EM, et al. The subcellular localization of GABA transporters and its implication for seizure management. Neurochem Res. 2015;40:410–9. - PubMed
-
- Dikow N, Maas B, Karch S, et al. 3p25.3 microdeletion of GABA transporters SLC6A1 and SLC6A11 results in intellectual disability, epilepsy and stereotypic behavior. Am J Med Genet A. 2014;164a:3061–8. - PubMed

