Expanded neurochemical profile in the early stage of Huntington disease using proton magnetic resonance spectroscopy
- PMID: 29315899
- PMCID: PMC5841244
- DOI: 10.1002/nbm.3880
Expanded neurochemical profile in the early stage of Huntington disease using proton magnetic resonance spectroscopy
Abstract
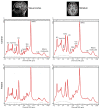
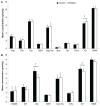
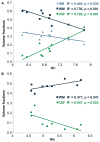
The striatum is a well-known region affected in Huntington disease (HD). However, other regions, including the visual cortex, are implicated. We have identified previously an abnormal energy response in the visual cortex of patients at an early stage of HD using 31 P magnetic resonance spectroscopy (31 P MRS). We therefore sought to further characterize these metabolic alterations with 1 H MRS using a well-validated semi-localized by adiabatic selective refocusing (semi-LASER) sequence that allows the measurement of an expanded number of neurometabolites. Ten early affected patients [Unified Huntington Disease Rating Scale (UHDRS), total motor score = 13.6 ± 10.8] and 10 healthy volunteers of similar age and body mass index (BMI) were recruited for the study. We performed 1 H MRS in the striatum - the region that is primarily affected in HD - and the visual cortex. The protocol allowed a reliable quantification of 10 metabolites in the visual cortex and eight in the striatum, compared with three to five metabolites in previous 1 H MRS studies performed in HD. We identified higher total creatine (p < 0.05) in the visual cortex and lower glutamate (p < 0.001) and total creatine (p < 0.05) in the striatum of patients with HD compared with controls. Less abundant neurometabolites [glutamine, γ-aminobutyric acid (GABA), glutathione, aspartate] showed similar concentrations in both groups. The protocol allowed the measurement of several additional metabolites compared with standard vendor protocols. Our study points to early changes in metabolites involved in energy metabolism in the visual cortex and striatum of patients with HD. Decreased striatal glutamate could reflect early neuronal dysfunction or impaired glutamatergic neurotransmission.
Keywords: 1H MRS; Huntington disease; movement disorders; neurochemical profile; neurometabolite; semi-LASER.
Copyright © 2018 John Wiley & Sons, Ltd.
Conflict of interest statement
Figures
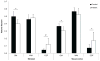
References
-
- Kremer B, Goldberg P, Andrew SE, et al. A worldwide study of the Huntington’s disease mutation. The sensitivity and specificity of measuring CAG repeats. N Engl J Med. 1994;330(20):1401–1406. - PubMed
-
- Tabrizi SJ, Scahill RI, Owen G, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. - PubMed
-
- Rosas HD, Koroshetz WJ, Chen YI, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60:1615–1620. - PubMed
-
- Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The Neuropathology of Huntington’s Disease. Curr Top Behav Neurosci. 2015;22:33–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

