Inhibition of VEGF-dependent angiogenesis and tumor angiogenesis by an optimized antibody targeting CLEC14a
- PMID: 29316206
- PMCID: PMC5830631
- DOI: 10.1002/1878-0261.12169
Inhibition of VEGF-dependent angiogenesis and tumor angiogenesis by an optimized antibody targeting CLEC14a
Abstract
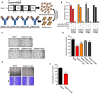
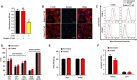
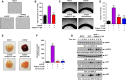
The C-type lectin-like domain of CLEC14a (CLEC14a-C-type lectin-like domain [CTLD]) is a key domain that mediates endothelial cell-cell contacts in angiogenesis. However, the role of CLEC14a-CTLD in pathological angiogenesis has not yet been clearly elucidated. In this study, through complementarity-determining region grafting, consecutive deglycosylation, and functional isolation, we generated a novel anti-angiogenic human monoclonal antibody that specifically targets CLEC14a-CTLD and that shows improved stability and homogeneity relative to the parental antibody. We found that this antibody directly inhibits CLEC14a-CTLD-mediated endothelial cell-cell contact and simultaneously downregulates expression of CLEC14a on the surface of endothelial cells. Using various in vitro and in vivo functional assays, we demonstrated that this antibody effectively suppresses vascular endothelial growth factor (VEGF)-dependent angiogenesis and tumor angiogenesis of SNU182 human hepatocellular carcinoma, CFPAC-1 human pancreatic cancer, and U87 human glioma cells. Furthermore, we also found that this antibody significantly inhibits tumor angiogenesis of HCT116 and bevacizumab-adapted HCT116 human colorectal cancer cells. These findings suggest that antibody targeting of CLEC14a-CTLD has the potential to suppress VEGF-dependent angiogenesis and tumor angiogenesis and that CLEC14a-CTLD may be a novel anti-angiogenic target for VEGF-dependent angiogenesis and tumor angiogenesis.
Keywords: CTLD; VEGF; CLEC14a; angiogenesis; tumor angiogenesis.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures



References
-
- Abel CA, Spiegelberg HL and Grey HM (1968) The carbohydrate contents of fragments and polypeptide chains of human gamma‐G‐myeloma proteins of different heavy‐chain subclasses. Biochemistry 7, 1271–1278. - PubMed
-
- Adamis AP, Aiello LP and D'Amato RA (1999) Angiogenesis and ophthalmic disease. Angiogenesis 3, 9–14. - PubMed
-
- Barbas CF (2001) Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Bessa J, Boeckle S, Beck H, Buckel T, Schlicht S, Ebeling M, Kiialainen A, Koulov A, Boll B, Weiser T et al (2015) The immunogenicity of antibody aggregates in a novel transgenic mouse model. Pharm Res 32, 2344–2359. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

