Crosstalk between hepatitis B virus X and high-mobility group box 1 facilitates autophagy in hepatocytes
- PMID: 29316268
- PMCID: PMC5830655
- DOI: 10.1002/1878-0261.12165
Crosstalk between hepatitis B virus X and high-mobility group box 1 facilitates autophagy in hepatocytes
Abstract
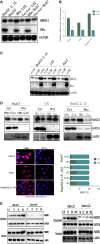
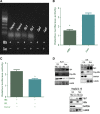
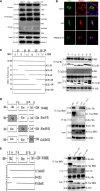
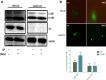
Hepatitis B virus (HBV) X (HBx) protein is a pivotal regulator of HBV-triggered autophagy. However, the role of HBx-induced epigenetic changes in autophagy remains largely unknown. The cytoplasmic (Cyt) high-mobility group box 1 (HMGB1) has been identified as a positive regulator of autophagy, and its Cyt translocation is closely associated with its acetylation status. Here, we evaluated the function of HMGB1 in HBx-mediated autophagy and its association with histone deacetylase (HDAC). Using cell lines with enforced expression of HBx, we demonstrated that HBx upregulated the expression of HMGB1 and promoted its Cyt translocation by acetylation to facilitate autophagy. We further identified the underlying mechanism by which decreased nuclear HDAC activity and expression levels contribute to the HBx-promoted hyperacetylation and subsequent translocation of HMGB1. We also identified the HDAC1 isoform as a critical factor in regulating this phenomenon. In addition, HBx bound to HMGB1 in the cytoplasm, which triggered autophagy in hepatocytes. Pharmacological inhibition of HMGB1 Cyt translocation with ethyl pyruvate prevented HBx-induced autophagy. These results demonstrate a novel function of acetylated HMGB1 in HBx-mediated autophagy in hepatocytes.
Keywords: acetylation; autophagy; hepatitis B virus; high-mobility group box 1; histone deacetylases; protein protein interaction.
© 2018 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures



References
-
- Barqasho B, Nowak P, Abdurahman S, Walther‐Jallow L and Sonnerborg A (2010) Implications of the release of high‐mobility group box 1 protein from dying cells during human immunodeficiency virus type 1 infection in vitro . J Gen Virol 91(Pt 7), 1800–1809. - PubMed
-
- Chen S, Dong Z, Yang P, Wang X, Jin G, Yu H, Chen L, Li L, Tang L, Bai S et al (2017) Hepatitis B virus X protein stimulates high mobility group box 1 secretion and enhances hepatocellular carcinoma metastasis. Cancer Lett 394, 22–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

