Exposure-Response Model of Subcutaneous C1-Inhibitor Concentrate to Estimate the Risk of Attacks in Patients With Hereditary Angioedema
- PMID: 29316335
- PMCID: PMC5869560
- DOI: 10.1002/psp4.12271
Exposure-Response Model of Subcutaneous C1-Inhibitor Concentrate to Estimate the Risk of Attacks in Patients With Hereditary Angioedema
Abstract
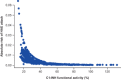
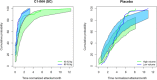
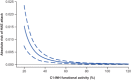
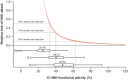
Subcutaneous C1-inhibitor (HAEGARDA, CSL Behring), is a US Food and Drug Administration (FDA)-approved, highly concentrated formulation of a plasma-derived C1-esterase inhibitor (C1-INH), which, in the phase III Clinical Studies for Optimal Management in Preventing Angioedema with Low-Volume Subcutaneous C1-inhibitor Replacement Therapy (COMPACT) trial, reduced the incidence of hereditary angioedema (HAE) attacks when given prophylactically. Data from the COMPACT trial were used to develop a repeated time-to-event model to characterize the timing and frequency of HAE attacks as a function of C1-INH activity, and then develop an exposure-response model to assess the relationship between C1-INH functional activity levels (C1-INH(f)) and the risk of an attack. The C1-INH(f) values of 33.1%, 40.3%, and 63.1% were predicted to correspond with 50%, 70%, and 90% reductions in the HAE attack risk, respectively, relative to no therapy. Based on trough C1-INH(f) values for the 40 IU/kg (40.2%) and 60 IU/kg (48.0%) C1-INH (SC) doses, the model predicted that 50% and 67% of the population, respectively, would see at least a 70% decrease in the risk of an attack.
© 2018 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
References
-
- Zuraw, B.L. Clinical practice. Hereditary angioedema. N. Engl. J. Med. 359, 1027–1036 (2008). - PubMed
-
- Toscani, M. & Riedl, M. Meeting the challenges and burdens associated with hereditary angioedema. Manag. Care 20, 44–51 (2011). - PubMed
-
- Zuraw, B.L. et al US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J. Allergy Clin. Immunol. Pract. 1, 458–467 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

