Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine
- PMID: 29316363
- PMCID: PMC5922416
- DOI: 10.1002/adhm.201700939
Exploiting Advanced Hydrogel Technologies to Address Key Challenges in Regenerative Medicine
Abstract
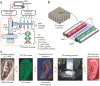
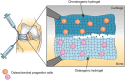
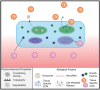
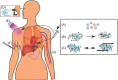
Regenerative medicine aims to tackle a panoply of challenges from repairing focal damage to articular cartilage to preventing pathological tissue remodeling after myocardial infarction. Hydrogels are water-swollen networks formed from synthetic or naturally derived polymers and are emerging as important tools to address these challenges. Recent advances in hydrogel chemistries are enabling researchers to create hydrogels that can act as 3D ex vivo tissue models, allowing them to explore fundamental questions in cell biology by replicating tissues' dynamic and nonlinear physical properties. Enabled by cutting edge techniques such as 3D bioprinting, cell-laden hydrogels are also being developed with highly controlled tissue-specific architectures, vasculature, and biological functions that together can direct tissue repair. Moreover, advanced in situ forming and acellular hydrogels are increasingly finding use as delivery vehicles for bioactive compounds and in mediating host cell response. Here, advances in the design and fabrication of hydrogels for regenerative medicine are reviewed. It is also addressed how controlled chemistries are allowing for precise engineering of spatial and time-dependent properties in hydrogels with a look to how these materials will eventually translate to clinical applications.
Keywords: advanced therapies; biomaterials; bioprinting; hydrogels; regenerative medicine; tissue engineering.
© 2018 The Authors. Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

