Active ingredients of a person-centred intervention for people on HIV treatment: analysis of mixed methods trial data
- PMID: 29316883
- PMCID: PMC5761128
- DOI: 10.1186/s12879-017-2900-0
Active ingredients of a person-centred intervention for people on HIV treatment: analysis of mixed methods trial data
Abstract
Background: A new model of care is required to meet the changing needs of people living with HIV (PLWH), particularly in low and middle-income countries, where prevalence is highest. We evaluated a palliative care intervention for PLWH in Mombasa, Kenya. Although we found no effect on pain (primary outcome), there was a positive effect on mental health (secondary outcome) in the intervention group. To inform replication and implementation, we have determined the active ingredients of the intervention and their mechanisms of action.
Methods: We conducted a randomised controlled trial (RCT) with qualitative exit interviews in HIV clinic attenders. The intervention was delivered over 5 months, with a minimum of 7 clinical contacts. Longitudinal quantitative data on components of care received were analysed using area under the curve and logistic regression. Qualitative data were analysed using inductive and deductive thematic analysis.
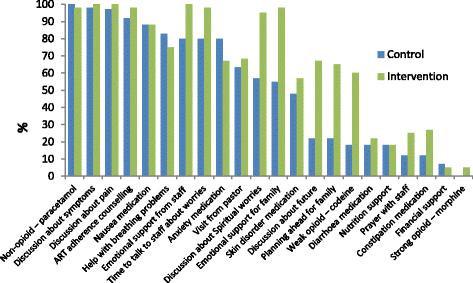
Results: Quantitative data analysis identified that intervention patients received more weak opioid, laxatives, discussion about spiritual worries, emotional support from staff for themselves and their families, time to talk about worries, discussion about future and planning ahead. Qualitative data analysis found that patients reported that having time to talk, appropriate pain medication and effective health education was of therapeutic value for their psychological well-being. Integration of mixed method findings suggest that positive effect in quantitative measures of mental health and well-being are attributable to the active ingredients of: appropriate medication, effective health education and counselling, and having time to talk in clinical encounters. Mechanisms of action include symptom relief, improved understanding of illness and treatment, and support focused on articulated concerns.
Conclusions: Routine care must provide opportunities and means for existing clinical staff to make routine appointments more person-centred. This approach enabled staff to identify and manage multidimensional problems and provide tailored health education and counselling.
Trial registration: ClinicalTrials.gov ( NCT01608802 ). Registered 12th May 2012.
Keywords: HIV; Mixed methods; Palliative care; Psychosocial; RCT.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been sought and secured from Kings College London Research Ethics Committee (BDM/10/11–31), and Kenyan Medical Research Institute (KEMRI/RES/7/3/1). All participants provided written informed consent to participate in the study, through a signature or thumbprint.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests. All authors were funded by their respective institutions for the manuscript preparation.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- UNAIDS . 90–90-90 an ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical