Use of an electronic medical record reminder improves HIV screening
- PMID: 29316919
- PMCID: PMC5761195
- DOI: 10.1186/s12913-017-2824-9
Use of an electronic medical record reminder improves HIV screening
Abstract
Background: More than 1 in 7 patients with human immunodeficiency virus (HIV) infection in the United States are unaware of their serostatus despite recommendations of US agencies that all adults through age 65 be screened for HIV at least once. To facilitate universal screening, an electronic medical record (EMR) reminder was created for our primary care practice. Screening rates before and after implementation were assessed to determine the impact of the reminder on screening rates.
Methods: A retrospective cohort analysis was performed for patients age 18-65 with visits between January 1, 2012-October 30, 2014. EMR databases were examined for HIV testing and selected patient characteristics. We evaluated the probability of HIV screening in unscreened patients before and after the reminder and used a multivariable generalized linear model to test the association between likelihood of HIV testing and specific patient characteristics.
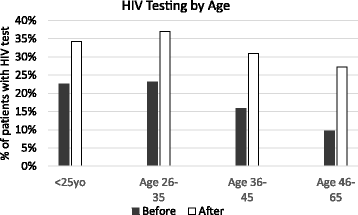
Results: Prior to the reminder, the probability of receiving an HIV test for previously unscreened patients was 15.3%. This increased to 30.7% after the reminder (RR 2.02, CI 1.95-2.09, p < 0.0001). The impact was most significant in patients age 45-65. White race, English as primary language, and higher median household income were associated with lower likelihoods of screening both before and after implementation (RR 0.68, CI 0.65-0.72; RR 0.74, CI 0.67-0.82; RR 0.84, CI 0.80-0.88, respectively).
Conclusions: The EMR reminder increased rates of HIV screening twofold in our practice. It was most effective in increasing screening rates in older patients. Patients who were white, English-speaking, and had higher incomes were less likely to be screened for HIV both before and after the reminder.
Keywords: Decision support; Electronic medical record reminder; HIV; Screening.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board on August 15, 2014 (protocol #2014P000210). Waiver of consent was requested due to the nature of the study as a de-identified retrospective chart review, and this was granted by the IRB.
Consent for publication
Not applicable
Competing interests
Authors CK, JT, GH, DB, GK, and LN declare that they have no competing interests. HL has consulted for the Medical Advisory Committee of Gilead Sciences and is employed by Up to Date.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Centers for Disease Control. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 U.S. dependent areas—2011. In: HIV Surveillance Supplemental Reports. CDC. 2013. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Accessed 9 Dec 2016.
-
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. - PubMed
-
- Human Immunodeficiency Virus (HIV) Infection: Screening. In: Recommendations for Primary Care Practice. United States Preventive Services Task Force. 2013. http://www.uspreventiveservicestaskforce.org/uspstf/uspshivi.htm. Accessed 21 Oct 2015.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

