HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): a randomised controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible: study protocol for a randomised controlled trial
- PMID: 29316962
- PMCID: PMC5761154
- DOI: 10.1186/s13063-017-2376-7
HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): a randomised controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible: study protocol for a randomised controlled trial
Abstract
Background: Osteoradionecrosis of the mandible is the most common serious complication of radiotherapy for head and neck malignancy. For decades, hyperbaric oxygen has been employed in efforts to prevent those cases of osteoradionecrosis that are precipitated by dental extractions or implant placement. The evidence for using hyperbaric oxygen remains poor and current clinical practice varies greatly. We describe a protocol for a clinical trial to assess the benefit of hyperbaric oxygen in the prevention of osteoradionecrosis during surgery on the irradiated mandible.
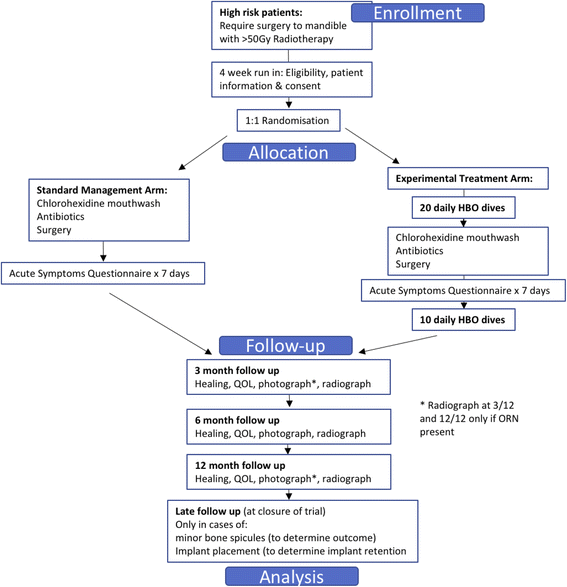
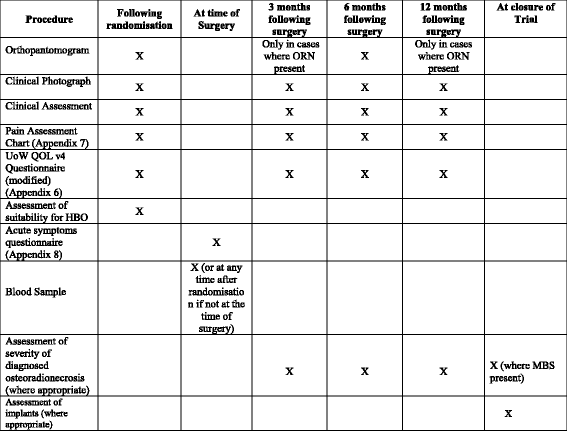
Methods/design: The HOPON trial is a phase III, randomised controlled, multi-centre trial. It employs an unblinded trial design, but the assessment of the primary endpoint, i.e. the diagnosis of osteoradionecrosis, is assessed on anonymised clinical photographs and radiographs by a blinded expert panel. Eligibility is through the need for a high-risk dental procedure in the mandible where at least 50-Gy radiotherapy has been received. Patients are randomised 1:1 to hyperbaric oxygen arm (Marx protocol) : control arm, but both groups receive antibiotics and chlorhexidine mouthwash. The primary endpoint is the presence of osteoradionecrosis at 6 months following surgery, but secondary endpoints include other time points, acute symptoms and pain, quality of life, and where implants are placed, their successful retention.
Discussion: The protocol presented has evolved through feasibility stages and through analysis of interim data. The classification of osteoradionecrosis has undergone technical refinement to ensure that robust definitions are employed. The HOPON trial is the only multi-centre RCT conducted in this clinical setting despite decades of use of hyperbaric oxygen for the prevention of osteoradionecrosis.
Trial registration: European Clinical Trials Database, ID: EudraCT200700622527 . First registered on 5 November 2007.
Conflict of interest statement
Authors’ information
Richard Shaw (corresponding author), Professor of Head and Neck/Oral and Maxillofacial Surgery, Mersey Head and Neck Oncology Research Group, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool and Aintree University Hospital NHS Foundation Trust. Christopher Butterworth, Consultant in Restorative Dentistry, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool, Aintree University Hospital NHS Foundation Trust and Liverpool University Dental Hospital. Binyam Tesfaye, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool. Matthew Bickerstaff, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool. Susanna Dodd, Department of Biostatistics, University of Liverpool. Gary Smerdon, Research Director, Devon Diseases Research Centre. Seema Chauhan, Operational Director, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool. Peter Brennan, Professor and Consultant in Oral and Maxillofacial Surgery, Portsmouth Hospitals NHS Trust; peter.brennan@porthosp.nhs.uk. Keith Webster, Consultant in Oral and Maxillofacial Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust. James McCaul, Professor and Consultant in Oral and Maxillofacial Surgery, Queen Elizabeth University Hospital, Glasgow. Peter Nixon, Consultant in Restorative Dentistry. Anastasias Kanatas, Professor and Consultant in Oral and Maxillofacial Surgery Leeds Dental Institute. Paul Silcocks, Trials Statistician, CRUK Liverpool Cancer Trials Unit, Department of Molecular and Clinical Cancer Medicine, University of Liverpool.
Ethics approval and consent to participate
The HOPON trial protocol has been granted ethical approval by Greater Manchester Central Research Ethics Committee (REC reference 08/H1008/32). All patients have been consented under this approval.
Consent for publication
Patient consent included the publication of all data.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

