The Diabetes Gene and Wnt Pathway Effector TCF7L2 Regulates Adipocyte Development and Function
- PMID: 29317436
- PMCID: PMC5860863
- DOI: 10.2337/db17-0318
The Diabetes Gene and Wnt Pathway Effector TCF7L2 Regulates Adipocyte Development and Function
Abstract
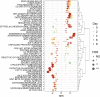
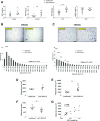
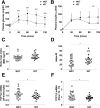
The gene encoding for transcription factor 7-like 2 (TCF7L2) is the strongest type 2 diabetes mellitus (T2DM) candidate gene discovered to date. The TCF7L2 protein is a key transcriptional effector of the Wnt/β-catenin signaling pathway, which is an important developmental pathway that negatively regulates adipogenesis. However, the precise role that TCF7L2 plays in the development and function of adipocytes remains largely unknown. Using a combination of in vitro approaches, we first show that TCF7L2 protein is increased during adipogenesis in 3T3-L1 cells and primary adipocyte stem cells and that TCF7L2 expression is required for the regulation of Wnt signaling during adipogenesis. Inactivation of TCF7L2 protein by removing the high-mobility group (HMG)-box DNA binding domain in mature adipocytes in vivo leads to whole-body glucose intolerance and hepatic insulin resistance. This phenotype is associated with increased subcutaneous adipose tissue mass, adipocyte hypertrophy, and inflammation. Finally, we demonstrate that TCF7L2 mRNA expression is downregulated in humans with impaired glucose tolerance and adipocyte insulin resistance, highlighting the translational potential of these findings. In summary, our data indicate that TCF7L2 has key roles in adipose tissue development and function that may reveal, at least in part, how TCF7L2 contributes to the pathophysiology of T2DM.
© 2018 by the American Diabetes Association.
Figures





References
-
- Jin T. Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocr Rev 2016;37:254–277 - PubMed
-
- Ross SE, Hemati N, Longo KA, et al. . Inhibition of adipogenesis by Wnt signaling. Science 2000;289:950–953 - PubMed
-
- Bennett CN, Ross SE, Longo KA, et al. . Regulation of Wnt signaling during adipogenesis. J Biol Chem 2002;277:30998–31004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

