Streptococcus pneumoniae Modulates Staphylococcus aureus Biofilm Dispersion and the Transition from Colonization to Invasive Disease
- PMID: 29317512
- PMCID: PMC5760742
- DOI: 10.1128/mBio.02089-17
Streptococcus pneumoniae Modulates Staphylococcus aureus Biofilm Dispersion and the Transition from Colonization to Invasive Disease
Abstract
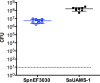
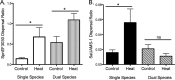
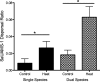
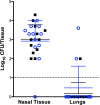
Streptococcus pneumoniae and Staphylococcus aureus are ubiquitous upper respiratory opportunistic pathogens. Individually, these Gram-positive microbes are two of the most common causative agents of secondary bacterial pneumonia following influenza A virus infection, and they constitute a significant source of morbidity and mortality. Since the introduction of the pneumococcal conjugate vaccine, rates of cocolonization with both of these bacterial species have increased, despite the traditional view that they are antagonistic and mutually exclusive. The interactions between S. pneumoniae and S. aureus in the context of colonization and the transition to invasive disease have not been characterized. In this report, we show that S. pneumoniae and S. aureus form stable dual-species biofilms on epithelial cells in vitro When these biofilms are exposed to physiological changes associated with viral infection, S. pneumoniae disperses from the biofilm, whereas S. aureus dispersal is inhibited. These findings were supported by results of an in vivo study in which we used a novel mouse cocolonization model. In these experiments, mice cocolonized in the nares with both bacterial species were subsequently infected with influenza A virus. The coinfected mice almost exclusively developed pneumococcal pneumonia. These results indicate that despite our previous report that S. aureus disseminates into the lungs of mice stably colonized with these bacteria following influenza A virus infection, cocolonization with S. pneumoniae in vitro and in vivo inhibits S. aureus dispersal and transition to disease. This study provides novel insight into both the interactions between S. pneumoniae and S. aureus during carriage and the transition from colonization to secondary bacterial pneumonia.IMPORTANCE In this study, we demonstrate that Streptococcus pneumoniae can modulate the pathogenic potential of Staphylococcus aureus in a model of secondary bacterial pneumonia. We report that host physiological signals related to viral infection cease to elicit a dispersal response from S. aureus while in a dual-species setting with S. pneumoniae, in direct contrast to results of previous studies with each species individually. This study underscores the importance of studying polymicrobial communities and their implications in disease states.
Keywords: Polymicrobial infections; Staphylococcus aureus; Streptococcus pneumoniae; secondary bacterial pneumonia.
Copyright © 2018 Reddinger et al.
Figures


Similar articles
-
Host Physiologic Changes Induced by Influenza A Virus Lead to Staphylococcus aureus Biofilm Dispersion and Transition from Asymptomatic Colonization to Invasive Disease.mBio. 2016 Aug 9;7(4):e01235-16. doi: 10.1128/mBio.01235-16. mBio. 2016. PMID: 27507829 Free PMC article.
-
Epidemiological Markers for Interactions Among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in Upper Respiratory Tract Carriage.J Infect Dis. 2016 May 15;213(10):1596-605. doi: 10.1093/infdis/jiv761. Epub 2015 Dec 23. J Infect Dis. 2016. PMID: 26704617 Free PMC article. Clinical Trial.
-
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection.mSphere. 2018 Aug 15;3(4):e00341-18. doi: 10.1128/mSphere.00341-18. mSphere. 2018. PMID: 30111629 Free PMC article.
-
Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: Myth or reality?Hum Vaccin Immunother. 2016;12(2):351-7. doi: 10.1080/21645515.2015.1081321. Hum Vaccin Immunother. 2016. PMID: 26905680 Free PMC article. Review.
-
Nasopharyngeal microbial interactions in the era of pneumococcal conjugate vaccination.Vaccine. 2013 May 1;31(19):2333-42. doi: 10.1016/j.vaccine.2013.03.024. Epub 2013 Mar 19. Vaccine. 2013. PMID: 23523773 Review.
Cited by
-
Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia.Front Immunol. 2018 Nov 16;9:2640. doi: 10.3389/fimmu.2018.02640. eCollection 2018. Front Immunol. 2018. PMID: 30505304 Free PMC article. Review.
-
Moraxella catarrhalis Promotes Stable Polymicrobial Biofilms With the Major Otopathogens.Front Microbiol. 2020 Jan 15;10:3006. doi: 10.3389/fmicb.2019.03006. eCollection 2019. Front Microbiol. 2020. PMID: 32010085 Free PMC article.
-
A Murine Model for Enhancement of Streptococcus pneumoniae Pathogenicity upon Viral Infection and Advanced Age.Infect Immun. 2021 Jul 15;89(8):e0047120. doi: 10.1128/IAI.00471-20. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 34031128 Free PMC article.
-
A Mouse Model for the Transition of Streptococcus pneumoniae from Colonizer to Pathogen upon Viral Co-Infection Recapitulates Age-Exacerbated Illness.J Vis Exp. 2022 Sep 28;(187):10.3791/64419. doi: 10.3791/64419. J Vis Exp. 2022. PMID: 36279528 Free PMC article.
-
Nasopharyngeal Carriage of Methicillin-Resistant Staphylococcus aureus (MRSA) among Sickle Cell Disease (SCD) Children in the Pneumococcal Conjugate Vaccine Era.Infect Dis Rep. 2021 Mar 1;13(1):191-204. doi: 10.3390/idr13010022. Infect Dis Rep. 2021. PMID: 33804397 Free PMC article.
References
-
- Huebner RE, Wasas AD, Klugman KP, Paediatric Study Group . 2000. Prevalence of nasopharyngeal antibiotic-resistant pneumococcal carriage in children attending private paediatric practices in Johannesburg. S Afr Med J 90:1116–1121. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

