Common basis for orofacial clefting and cortical interneuronopathy
- PMID: 29317601
- PMCID: PMC5802454
- DOI: 10.1038/s41398-017-0057-7
Common basis for orofacial clefting and cortical interneuronopathy
Abstract
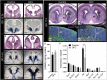
Orofacial clefts (OFCs) of the lip and/or palate are among the most common human birth defects. Current treatment strategies focus on functional and cosmetic repair but even when this care is available, individuals born with OFCs are at high risk for persistent neurobehavioral problems. In addition to learning disabilities and reduced academic achievement, recent evidence associates OFCs with elevated risk for a constellation of psychiatric outcomes including anxiety disorders, autism spectrum disorder, and schizophrenia. The relationship between these outcomes and OFCs is poorly understood and controversial. Recent neuroimaging studies in humans and mice demonstrate subtle morphological brain abnormalities that co-occur with OFCs but specific molecular and cellular mechanisms have not been investigated. Here, we provide the first evidence directly linking OFC pathogenesis to abnormal development of GABAergic cortical interneurons (cINs). Lineage tracing revealed that the structures that form the upper lip and palate develop in molecular synchrony and spatiotemporal proximity to cINs, suggesting these populations may have shared sensitivity to genetic and/or teratogenic insult. Examination of cIN development in a mouse model of nonsyndromic OFCs revealed significant disruptions in cIN proliferation and migration, culminating in misspecification of the somatostatin-expressing subgroup. These findings reveal a unified developmental basis for orofacial clefting and disrupted cIN development, and may explain the significant overlap in neurobehavioral and psychiatric outcomes associated with OFCs and cIN dysfunction. This emerging mechanistic understanding for increased prevalence of adverse neurobehavioral outcomes in OFC patients is the entry-point for developing evidence-based therapies to improve patient outcomes.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- Waitzman N., Scheffler R. M. & Romano P. S. The Cost of Birth Defects: Estimates of the Value of Prevention, xiv (University Press of America: Lanham, MD), 1996, 262pp.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

