Nanoparticle elasticity directs tumor uptake
- PMID: 29317633
- PMCID: PMC5760638
- DOI: 10.1038/s41467-017-02588-9
Nanoparticle elasticity directs tumor uptake
Abstract
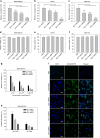
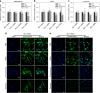
To date, the role of elasticity in drug delivery remains elusive due to the inability to measure microscale mechanics and alter rheology without affecting chemistry. Herein, we describe the in vitro cellular uptake and in vivo tumor uptake of nanolipogels (NLGs). NLGs are composed of identical lipid bilayers encapsulating an alginate core, with tunable elasticity. The elasticity of NLGs was evaluated by atomic force microscopy, which demonstrated that they exhibit Young's moduli ranging from 45 ± 9 to 19,000 ± 5 kPa. Neoplastic and non-neoplastic cells exhibited significantly greater uptake of soft NLGs (Young's modulus <1.6 MPa) relative to their elastic counterparts (Young's modulus >13.8 MPa). In an orthotopic breast tumor model, soft NLGs accumulated significantly more in tumors, whereas elastic NLGs preferentially accumulated in the liver. Our findings demonstrate that particle elasticity directs tumor accumulation, suggesting that it may be a design parameter to enhance tumor delivery efficiency.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

