Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease
- PMID: 29317695
- PMCID: PMC5760709
- DOI: 10.1038/s41598-017-18405-8
Alterations in the properties of the cell membrane due to glycosphingolipid accumulation in a model of Gaucher disease
Abstract
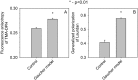
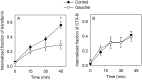
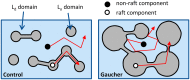
Gaucher disease is a lysosomal storage disease characterized by the malfunction of glucocerebrosidase resulting in the accumulation of glucosylceramide and other sphingolipids in certain cells. Although the disease symptoms are usually attributed to the storage of undigested substrate in lysosomes, here we show that glycosphingolipids accumulating in the plasma membrane cause profound changes in the properties of the membrane. The fluidity of the sphingolipid-enriched membrane decreased accompanied by the enlargement of raft-like ordered membrane domains. The mobility of non-raft proteins and lipids was severely restricted, while raft-resident components were only mildly affected. The rate of endocytosis of transferrin receptor, a non-raft protein, was significantly retarded in Gaucher cells, while the endocytosis of the raft-associated GM1 ganglioside was unaffected. Interferon-γ-induced STAT1 phosphorylation was also significantly inhibited in Gaucher cells. Atomic force microscopy revealed that sphingolipid accumulation was associated with a more compliant membrane capable of producing an increased number of nanotubes. The results imply that glycosphingolipid accumulation in the plasma membrane has significant effects on membrane properties, which may be important in the pathogenesis of Gaucher disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Grabowski GA. Gaucher disease and other storage disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:13–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

